Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats
- PMID: 11331393
- PMCID: PMC6762466
- DOI: 10.1523/JNEUROSCI.21-10-03639.2001
Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats
Abstract
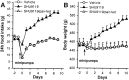
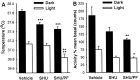
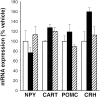
The CNS melanocortin (MC) system is implicated as a mediator of the central effects of leptin, and reduced activity of the CNS MC system promotes obesity in both rodents and humans. Because activation of CNS MC receptors has direct effects on autonomic outflow and metabolism, we hypothesized that food intake-independent mechanisms contribute to development of obesity induced by pharmacological blockade of MC receptors in the brain and that changes in hypothalamic neuropeptidergic systems known to regulate weight gain [i.e., corticotropin-releasing hormone (CRH), cocaine-amphetamine-related transcript (CART), proopiomelanocortin (POMC), and neuropeptide Y (NPY)] would trigger this effect. Relative to vehicle-treated controls, third intracerebroventricular (i3vt) administration of the MC receptor antagonist SHU9119 to rats for 11 d doubled food and water intake (toward the end of treatment) and increased body weight ( approximately 14%) and fat content ( approximately 90%), hepatic glycogen content ( approximately 40%), and plasma levels of cholesterol ( approximately 48%), insulin ( approximately 259%), glucagon ( approximately 80%), and leptin ( approximately 490%), whereas spontaneous locomotor activity and body temperature were reduced. Pair-feeding of i3vt SHU9119-treated animals to i3vt vehicle-treated controls normalized plasma levels of insulin, glucagon, and hepatic glycogen content, but only partially reversed the elevations of plasma cholesterol ( approximately 31%) and leptin ( approximately 104%) and body fat content ( approximately 27%). Reductions in body temperature and locomotor activity induced by i3vt SHU9119 were not reversed by pair feeding, but rather were more pronounced. None of the effects found can be explained by peripheral action of the compound. The obesity effects occurred despite a lack in neuropeptide expression responses in the neuroanatomical range selected across the arcuate (i.e., CART, POMC, and NPY) and paraventricular (i.e., CRH) hypothalamus. The results indicate that reduced activity of the CNS MC pathway promotes fat deposition via both food intake-dependent and -independent mechanisms.
Figures
References
-
- Boston BA, Blaydon KM, Varnerin J, Cone RD. Independent and additive effects of central POMC and leptin pathways on murine obesity. Science. 1997;28:1641–1644. - PubMed
-
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. - PubMed
-
- Buwalda B, de Boer SF, Van Kalkeren AA, Koolhaas JM. Physiological and behavioral effects of chronic intracerebroventricular infusion of corticotropin-releasing factor in the rat. Psychoneuroendocrinology. 1997;22:297–309. - PubMed
-
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central networks. Science. 1995;269:546–549. - PubMed
-
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan X-M, Yu H, Rosemblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg HT. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
