Susceptibility to kindling and neuronal connections of the anterior claustrum
- PMID: 11331397
- PMCID: PMC6762482
- DOI: 10.1523/JNEUROSCI.21-10-03674.2001
Susceptibility to kindling and neuronal connections of the anterior claustrum
Abstract
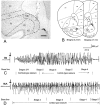
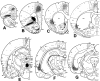
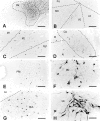
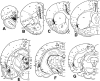
The claustrum has been implicated in the kindling of generalized seizures from limbic sites. We examined the susceptibility of the anterior claustrum itself to kindling and correlated this with an anatomical investigation of its afferent and efferent connections. Electrical stimulation of the anterior claustrum resulted in a pattern of rapid kindling with two distinct phases. Early kindling involved extremely rapid progression to bilaterally generalized seizures of short duration. With repeated daily kindling stimulations, early-phase generalized seizures abruptly became more elaborate and prolonged, resembling limbic-type seizures as triggered from the amygdala. We suggest that the rapid rate of kindling from the anterior claustrum is an indication that the claustrum is functionally close to the mechanisms of seizure generalization. In support of our hypothesis, we found significant afferent, efferent, and often reciprocal connections between the anterior claustrum and areas that have been implicated in the generation of generalized seizures, including frontal and motor cortex, limbic cortex, amygdala, and endopiriform nucleus. Additional connections were found with various other structures, including olfactory areas, nucleus accumbens, midline thalamus, and brainstem nuclei including the substantia nigra and the dorsal raphe nucleus. The anatomical connections of the anterior claustrum are consistent with its very high susceptibility to kindling and support the view that the claustrum is part of a forebrain network of structures participating in the generalization of seizures.
Figures


Similar articles
-
Claustral lesions delay amygdaloid kindling in the rat.Epilepsia. 2000 Sep;41(9):1095-101. doi: 10.1111/j.1528-1157.2000.tb00313.x. Epilepsia. 2000. PMID: 10999547
-
Further evidence for a role of the anterior claustrum in epileptogenesis.Neuroscience. 2004;125(1):57-62. doi: 10.1016/j.neuroscience.2004.01.043. Neuroscience. 2004. PMID: 15051145
-
Kindling of claustrum and insular cortex: comparison to perirhinal cortex in the rat.Eur J Neurosci. 2001 Apr;13(8):1501-19. doi: 10.1046/j.0953-816x.2001.01532.x. Eur J Neurosci. 2001. PMID: 11328345
-
The role of the piriform cortex in kindling.Prog Neurobiol. 1996 Dec;50(5-6):427-81. doi: 10.1016/s0301-0082(96)00036-6. Prog Neurobiol. 1996. PMID: 9015822 Review.
-
Subcortical structures and pathways involved in convulsive seizure generation.J Clin Neurophysiol. 1992 Apr;9(2):264-77. doi: 10.1097/00004691-199204010-00007. J Clin Neurophysiol. 1992. PMID: 1350593 Review.
Cited by
-
Human Claustrum Connections: Robust In Vivo Detection by DWI-Based Tractography in Two Large Samples.Hum Brain Mapp. 2024 Oct;45(14):e70042. doi: 10.1002/hbm.70042. Hum Brain Mapp. 2024. PMID: 39397271 Free PMC article.
-
The piriform cortex and human focal epilepsy.Front Neurol. 2014 Dec 8;5:259. doi: 10.3389/fneur.2014.00259. eCollection 2014. Front Neurol. 2014. PMID: 25538678 Free PMC article. Review.
-
Interhemispheric connections between the infralimbic and entorhinal cortices: The endopiriform nucleus has limbic connections that parallel the sensory and motor connections of the claustrum.J Comp Neurol. 2017 Apr 15;525(6):1363-1380. doi: 10.1002/cne.23981. Epub 2016 Feb 24. J Comp Neurol. 2017. PMID: 26860547 Free PMC article.
-
Is there room in epilepsy for the claustrum?Front Syst Biol. 2024 Apr 3;4:1385112. doi: 10.3389/fsysb.2024.1385112. eCollection 2024. Front Syst Biol. 2024. PMID: 40809137 Free PMC article.
-
The Claustrum in Relation to Seizures and Electrical Stimulation.Front Neuroanat. 2019 Feb 12;13:8. doi: 10.3389/fnana.2019.00008. eCollection 2019. Front Neuroanat. 2019. PMID: 30809132 Free PMC article. Review.
References
-
- Bayer SA, Altman J. Development of the endopiriform nucleus and the claustrum in the rat brain. Neuroscience. 1991;45:391–412. - PubMed
-
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. - PubMed
-
- Beckstead RM. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol. 1979;184:43–61. - PubMed
-
- Behan M, Haberly LB. Intrinsic and efferent connections of the endopiriform nucleus in rat. J Comp Neurol. 1999;408:532–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
