Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen
- PMID: 11331578
- PMCID: PMC125246
- DOI: 10.1093/emboj/20.9.2120
Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen
Abstract
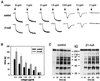
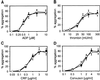
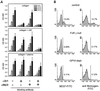
Platelet adhesion on and activation by components of the extracellular matrix are crucial to arrest post-traumatic bleeding, but can also harm tissue by occluding diseased vessels. Integrin alpha2beta1 is thought to be essential for platelet adhesion to subendothelial collagens, facilitating subsequent interactions with the activating platelet collagen receptor, glycoprotein VI (GPVI). Here we show that Cre/loxP-mediated loss of beta1 integrin on platelets has no significant effect on the bleeding time in mice. Aggregation of beta1-null platelets to native fibrillar collagen is delayed, but not reduced, whereas aggregation to enzymatically digested soluble collagen is abolished. Furthermore, beta1-null platelets adhere to fibrillar, but not soluble collagen under static as well as low (150 s(-1)) and high (1000 s(-1)) shear flow conditions, probably through binding of alphaIIbbeta3 to von Willebrand factor. On the other hand, we show that platelets lacking GPVI can not activate integrins and consequently fail to adhere to and aggregate on fibrillar as well as soluble collagen. These data show that GPVI plays the central role in platelet-collagen interactions by activating different adhesive receptors, including alpha2beta1 integrin, which strengthens adhesion without being essential.
Figures
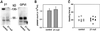




References
-
- Arai M., Yamamoto,N., Moroi,M., Akamatsu,N., Fukutake,K. and Tanoue,K. (1995) Platelets with 10% of the normal amount of glycoprotein VI have an impaired response to collagen that results in a mild bleeding tendency. Br. J. Haematol., 89, 124–130. - PubMed
-
- Asselin J., Gibbins,J.M., Achison,M., Lee,Y.H., Morton,L.F., Farndale,R.W., Barnes,M.J. and Watson,S.P. (1997) A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase C γ2 in platelets independent of the integrin α2β1. Blood, 89, 1235–1242. - PubMed
-
- Barnes M.J., Knight,C.G. and Farndale,R.W. (1998) The collagen– platelet interaction. Curr. Opin. Hematol., 5, 314–320. - PubMed
-
- Baumgartner H.R. (1977) Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with α chymotrypsin-digested subendothelium. Thromb. Haemost., 37, 1–16. - PubMed
-
- Bergmeier W., Rackebrandt,K., Schroder,W., Zirngibl,H. and Nieswandt,B. (2000) Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood, 95, 886–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

