PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic
- PMID: 11331585
- PMCID: PMC125242
- DOI: 10.1093/emboj/20.9.2191
PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic
Abstract
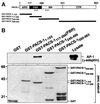
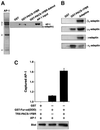
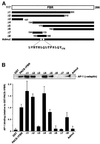
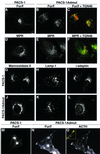
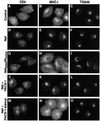
PACS-1 is a cytosolic protein involved in controlling the correct subcellular localization of integral membrane proteins that contain acidic cluster sorting motifs, such as furin and human immunodeficiency virus type 1 (HIV-1) NEF: We have now investigated the interaction of PACS-1 with heterotetrameric adaptor complexes. PACS-1 associates with both AP-1 and AP-3, but not AP-2, and forms a ternary complex between furin and AP-1. A short sequence within PACS-1 that is essential for binding to AP-1 has been identified. Mutation of this motif yielded a dominant-negative PACS-1 molecule that can still bind to acidic cluster motifs on cargo proteins but not to adaptor complexes. Expression of dominant-negative PACS-1 causes a mislocalization of both furin and mannose 6-phosphate receptor from the trans-Golgi network, but has no effect on the localization of proteins that do not contain acidic cluster sorting motifs. Furthermore, expression of dominant-negative PACS-1 inhibits the ability of HIV-1 Nef to downregulate MHC-I. These studies demonstrate the requirement for PACS-1 interactions with adaptor proteins in multiple processes, including secretory granule biogenesis and HIV-1 pathogenesis.
Figures




References
-
- Anderson E.D., Thomas,L., Hayflick,J.S. and Thomas,G. (1993) Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J. Biol. Chem., 268, 24887–24891. - PubMed
-
- Austin C., Hinners,I. and Tooze,S.A. (2000) Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem., 275, 21862–21869. - PubMed
-
- Chen H.J., Yuan,J. and Lobel,P. (1997) Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J. Biol. Chem., 272, 7003–7012. - PubMed
-
- Dell’Angelica E.C., Mullins,C. and Bonifacino,J.S. (1999) AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem., 274, 7278–7285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

