SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis
- PMID: 11331586
- PMCID: PMC125434
- DOI: 10.1093/emboj/20.9.2202
SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis
Abstract
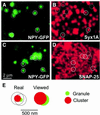
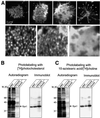
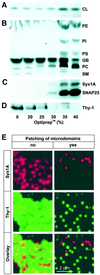
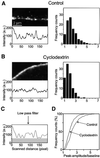
During exocytosis, SNARE proteins of secretory vesicles interact with the corresponding SNARE proteins in the plasmalemma to initiate the fusion reaction. However, it is unknown whether SNAREs are uniformly distributed in the membrane or whether specialized fusion sites exist. Here we report that in the plasmalemma, syntaxins are concentrated in 200 nm large, cholesterol-dependent clusters at which secretory vesicles preferentially dock and fuse. The syntaxin clusters are distinct from cholesterol-dependent membrane rafts since they are Triton X-100-soluble and do not co-patch with raft markers. Synaptosomal-associated protein (SNAP)-25 is also clustered in spots, which partially overlap with syntaxin. Cholesterol depletion causes dispersion of these clusters, which is associated with a strong reduction in the rate of secretion, whereas the characteristics of individual exocytic events are unchanged. This suggests that high local concentrations of SNAREs are required for efficient fusion.
Figures



Similar articles
-
Effect of cholesterol depletion on exocytosis of alveolar type II cells.Am J Respir Cell Mol Biol. 2006 Jun;34(6):677-87. doi: 10.1165/rcmb.2005-0418OC. Epub 2006 Jan 26. Am J Respir Cell Mol Biol. 2006. PMID: 16439800 Free PMC article.
-
Ternary SNARE complexes are enriched in lipid rafts during mast cell exocytosis.Traffic. 2006 Nov;7(11):1482-94. doi: 10.1111/j.1600-0854.2006.00490.x. Epub 2006 Sep 19. Traffic. 2006. PMID: 16984405
-
Plasma membrane targeting of exocytic SNARE proteins.Biochim Biophys Acta. 2004 Aug 23;1693(2):81-9. doi: 10.1016/j.bbamcr.2004.05.008. Biochim Biophys Acta. 2004. PMID: 15313010 Review.
-
Sequential exocytosis of insulin granules is associated with redistribution of SNAP25.J Cell Biol. 2004 Apr 26;165(2):255-62. doi: 10.1083/jcb.200312033. J Cell Biol. 2004. PMID: 15117968 Free PMC article.
-
SNAREs and SNARE regulators in membrane fusion and exocytosis.Cell Mol Life Sci. 1999 May;55(5):707-34. doi: 10.1007/s000180050328. Cell Mol Life Sci. 1999. PMID: 10379359 Free PMC article. Review.
Cited by
-
Organization and dynamics of SNARE proteins in the presynaptic membrane.Front Physiol. 2015 Mar 19;6:89. doi: 10.3389/fphys.2015.00089. eCollection 2015. Front Physiol. 2015. PMID: 25852575 Free PMC article. Review.
-
Roles of cholesterol in vesicle fusion and motion.Biophys J. 2009 Sep 2;97(5):1371-80. doi: 10.1016/j.bpj.2009.06.025. Biophys J. 2009. PMID: 19720025 Free PMC article.
-
Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion.EMBO J. 2007 Dec 12;26(24):4935-45. doi: 10.1038/sj.emboj.7601915. Epub 2007 Nov 15. EMBO J. 2007. PMID: 18007597 Free PMC article.
-
Correlation of syntaxin-1 and SNAP-25 clusters with docking and fusion of insulin granules analysed by total internal reflection fluorescence microscopy.Diabetologia. 2004 Dec;47(12):2200-7. doi: 10.1007/s00125-004-1579-0. Epub 2004 Dec 11. Diabetologia. 2004. PMID: 15647897
-
Mechanical coupling via the membrane fusion SNARE protein syntaxin 1A: a molecular dynamics study.Biophys J. 2003 Mar;84(3):1527-47. doi: 10.1016/S0006-3495(03)74965-0. Biophys J. 2003. PMID: 12609859 Free PMC article.
References
-
- Aguado F., Majo,G., Ruiz-Montasell,B., Canals,J.M., Casanova,A., Marsal,J. and Blasi,J. (1996) Expression of synaptosomal-associated protein SNAP-25 in endocrine anterior pituitary cells. Eur. J. Cell Biol., 69, 351–359. - PubMed
-
- Angleson J.K., Cochilla,A.J., Kilic,G., Nussinovitch,I. and Betz,W.J. (1999) Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nature Neurosci., 2, 440–446. - PubMed
-
- Barnstable C.J., Hofstein,R. and Akagawa,K. (1985) A marker of early amacrine cell development in rat retina. Brain Res., 352, 286–290. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

