The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana
- PMID: 11331608
- PMCID: PMC312681
- DOI: 10.1101/gad.200201
The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana
Abstract
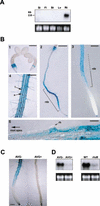
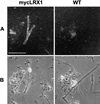
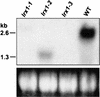
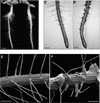
In plants, the cell wall is a major determinant of cell morphogenesis. Cell enlargement depends on the tightly regulated expansion of the wall, which surrounds each cell. However, the qualitative and quantitative mechanisms controlling cell wall enlargement are still poorly understood. Here, we report the molecular and functional characterization of LRX1, a new Arabidopsis gene that encodes a chimeric leucine-rich repeat/extensin protein. LRX1 is expressed in root hair cells and the protein is specifically localized in the wall of the hair proper, where it becomes insolubilized during development. lrx1-null mutants, isolated by a reverse-genetic approach, develop root hairs that frequently abort, swell, or branch. Complementation and overexpression experiments using modified LRX1 proteins indicate that the interaction with the cell wall is important for LRX1 function. These results suggest that LRX1 is an extracellular component of a mechanism regulating root hair morphogenesis and elongation by controlling either polarized growth or cell wall formation and assembly.
Figures


References
-
- Baumann E, Lewald J, Saedler H, Schulz B, Wisman E. Successful PCR-based reverse genetic screens using an En-1–mutagenised Arabidopsis thaliana population generated via single-seed descent. Theor Appl Genet. 1998;97:729–734.
-
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol. 1992;20:1195–1197. - PubMed
-
- Bibikova TN, Zhigilei A, Gilroy S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 1997;203:495–505. - PubMed
-
- Bibikova TN, Blancaflor EB, Gilroy S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999;17:657–665. - PubMed
-
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: A novel, rapid defense response. Cell. 1992;70:21–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
