Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning
- PMID: 11331610
- PMCID: PMC312687
- DOI: 10.1101/gad.191301
Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning
Abstract
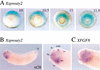
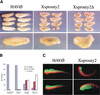
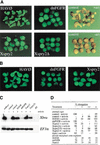
Signal transduction through the FGF receptor is essential for the specification of the vertebrate body plan. Blocking the FGF pathway in early Xenopus embryos inhibits mesoderm induction and results in truncation of the anterior-posterior axis. The Drosophila gene sprouty encodes an antagonist of FGF signaling, which is transcriptionally induced by the pathway, but whose molecular functions are poorly characterized. We have cloned Xenopus sprouty2 and show that it is expressed in a similar pattern to known FGFs and is dependent on the FGF/Ras/MAPK pathway for its expression. Overexpression of Xsprouty2 in both embryos and explant assays results in the inhibition of the cell movements of convergent extension. Although blocking FGF/Ras/MAPK signaling leads to an inhibition of mesodermal gene expression, these markers are unaffected by Xsprouty2, indicating that mesoderm induction and patterning occurs normally in these embryos. Finally, using Xenopus oocytes we show that Xsprouty2 is an intracellular antagonist of FGF-dependent calcium signaling. These results provide evidence for at least two distinct FGF-dependent signal transduction pathways: a Sprouty-insensitive Ras/MAPK pathway required for the transcription of most mesodermal genes, and a Sprouty-sensitive pathway required for coordination of cellular morphogenesis.
Figures



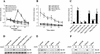
Similar articles
-
Ras-mediated FGF signaling is required for the formation of posterior but not anterior neural tissue in Xenopus laevis.Dev Biol. 2000 Nov 1;227(1):183-96. doi: 10.1006/dbio.2000.9889. Dev Biol. 2000. PMID: 11076686
-
Expression of activated MAP kinase in Xenopus laevis embryos: evaluating the roles of FGF and other signaling pathways in early induction and patterning.Dev Biol. 2000 Dec 1;228(1):41-56. doi: 10.1006/dbio.2000.9917. Dev Biol. 2000. PMID: 11087625
-
Expression cloning of Xenopus Os4, an evolutionarily conserved gene, which induces mesoderm and dorsal axis.Dev Biol. 2001 Nov 1;239(1):118-31. doi: 10.1006/dbio.2001.0420. Dev Biol. 2001. PMID: 11784023
-
The role of fibroblast growth factors in early Xenopus development.Biochem Soc Symp. 1996;62:1-12. Biochem Soc Symp. 1996. PMID: 8971335 Review.
-
Genetic evidence for posterior specification by convergent extension in the Xenopus embryo.Dev Growth Differ. 1998 Apr;40(2):125-32. doi: 10.1046/j.1440-169x.1998.00002.x. Dev Growth Differ. 1998. PMID: 9572355 Review.
Cited by
-
Characterization of mouse Rsk4 as an inhibitor of fibroblast growth factor-RAS-extracellular signal-regulated kinase signaling.Mol Cell Biol. 2004 May;24(10):4255-66. doi: 10.1128/MCB.24.10.4255-4266.2004. Mol Cell Biol. 2004. PMID: 15121846 Free PMC article.
-
Identification of a new Sprouty protein responsible for the inhibition of the Bombyx mori nucleopolyhedrovirus reproduction.PLoS One. 2014 Jun 10;9(6):e99200. doi: 10.1371/journal.pone.0099200. eCollection 2014. PLoS One. 2014. PMID: 24915434 Free PMC article.
-
Early coordination of cell migration and cardiac fate determination during mammalian gastrulation.EMBO J. 2025 Jun;44(12):3327-3359. doi: 10.1038/s44318-025-00441-0. Epub 2025 May 13. EMBO J. 2025. PMID: 40360834 Free PMC article.
-
Fibroblast growth factor (FGF) signaling in development and skeletal diseases.Genes Dis. 2014 Dec 1;1(2):199-213. doi: 10.1016/j.gendis.2014.09.005. Genes Dis. 2014. PMID: 25679016 Free PMC article.
-
Wnt-frizzled planar cell polarity signaling in the regulation of cell motility.Curr Top Dev Biol. 2022;150:255-297. doi: 10.1016/bs.ctdb.2022.03.006. Epub 2022 May 25. Curr Top Dev Biol. 2022. PMID: 35817505 Free PMC article. Review.
References
-
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. - PubMed
-
- Amaya E. FGF signalling in early Xenopus development. Phd Thesis. San Francisco: University of California; 1992.
-
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. - PubMed
-
- Beiman M, Shilo BZ, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes & Dev. 1996;10:2993–3002. - PubMed
-
- Carballada R, Yasuo H, Lemaire P. Phosphatidylinositol-3 kinase acts in parallel to the ERK MAP kinase in the FGF pathway during Xenopus mesoderm induction. Development. 2001;128:35–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
