Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression
- PMID: 11333291
- PMCID: PMC4374741
- DOI: 10.1093/jnci/93.9.691
Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression
Abstract
Background: The recently identified RASSF1 locus is located within a 120-kilobase region of chromosome 3p21.3 that frequently undergoes allele loss in lung and breast cancers. We explored the hypothesis that RASSF1 encodes a tumor suppressor gene for lung and breast cancers.
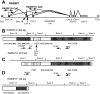
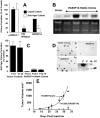
Methods: We assessed expression of two RASSF1 gene products, RASSF1A and RASSF1C, and the methylation status of their respective promoters in 27 non-small-cell lung cancer (NSCLC) cell lines, in 107 resected NSCLCs, in 47 small-cell lung cancer (SCLC) cell lines, in 22 breast cancer cell lines, in 39 resected breast cancers, in 104 nonmalignant lung samples, and in three breast and lung epithelial cultures. We also transfected a lung cancer cell line that lacks RASSF1A expression with vectors containing RASSF1A complementary DNA to determine whether exogenous expression of RASSF1A would affect in vitro growth and in vivo tumorigenicity of this cell line. All statistical tests were two-sided.
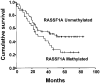
Results: RASSF1A messenger RNA was expressed in nonmalignant epithelial cultures but not in 100% of the SCLC, in 65% of the NSCLC, or in 60% of the breast cancer lines. By contrast, RASSF1C was expressed in all nonmalignant cell cultures and in nearly all cancer cell lines. RASSF1A promoter hypermethylation was detected in 100% of SCLC, in 63% of NSCLC, in 64% of breast cancer lines, in 30% of primary NSCLCs, and in 49% of primary breast tumors but in none of the nonmalignant lung tissues. RASSF1A promoter hypermethylation in resected NSCLCs was associated with impaired patient survival (P =.046). Exogenous expression of RASSF1A in a cell line lacking expression decreased in vitro colony formation and in vivo tumorigenicity.
Conclusion: RASSF1A is a potential tumor suppressor gene that undergoes epigenetic inactivation in lung and breast cancers through hypermethylation of its promoter region.
Figures



Comment in
-
Promoter hypermethylation--can this change alone ever designate true tumor suppressor gene function?J Natl Cancer Inst. 2001 May 2;93(9):664-5. doi: 10.1093/jnci/93.9.664. J Natl Cancer Inst. 2001. PMID: 11333278 No abstract available.
References
-
- Wistuba I, Behrens C, Virmani A, Mele G, Milchgrub S, Girard L, et al. High resolution chromosome 3p allelotyping of human lung cancer and preneoplastic/preinvasive bronchial epithelium reveals multiple, discontinuous sites of 3p allele loss and three regions of frequent breakpoints. Cancer Res. 2000;60:1949–60. - PubMed
-
- Sekido Y, Fong KM, Minna JD. Progress in understanding the molecular pathogenesis of human lung cancer. Biochim Biophys Acta. 1998;1378:F21–59. - PubMed
-
- Wistuba II, Behrens C, Milchgrub S, Bryant D, Hung J, Minna JD, et al. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene. 1999;18:643–50. - PubMed
-
- Kok K, Naylor SL, Buys CH. Deletions of the short arm of chromosome 3 in solid tumors and the search for suppressor genes. Adv Cancer Res. 1997;71:27–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

