Adaptation of chimeric retroviruses in vitro and in vivo: isolation of avian retroviral vectors with extended host range
- PMID: 11333876
- PMCID: PMC114900
- DOI: 10.1128/JVI.75.11.4973-4983.2001
Adaptation of chimeric retroviruses in vitro and in vivo: isolation of avian retroviral vectors with extended host range
Abstract
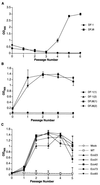
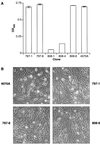
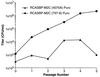
We have designed and characterized two new replication-competent avian sarcoma/leukosis virus-based retroviral vectors with amphotropic and ecotropic host ranges. The amphotropic vector RCASBP-M2C(797-8), was obtained by passaging the chimeric retroviral vector RCASBP-M2C(4070A) (6) in chicken embryos. The ecotropic vector, RCASBP(Eco), was created by replacing the env-coding region in the retroviral vector RCASBP(A) with the env region from an ecotropic murine leukemia virus. It replicates efficiently in avian DFJ8 cells that express murine ecotropic receptor. For both vectors, permanent cell lines that produce viral stocks with titers of about 5 x 10(6) CFU/ml on mammalian cells can be easily established by passaging transfected avian cells. Some chimeric viruses, for example, RCASBP(Eco), replicate efficiently without modifications. For those chimeric viruses that do require modification, adaptation by passage in vitro or in vivo is a general strategy. This strategy has been used to prepare vectors with altered host range and could potentially be used to develop vectors that would be useful for targeted gene delivery.
Figures



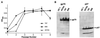

References
-
- Ablashi D V, Armstrong G R, Turner W. Production and characterization of human cell-adapted murine Rauscher virus pseudotype of murine sarcoma virus. J Natl Cancer Inst. 1973;50:381–385. - PubMed
-
- Al-Adhami R, Chapman A L. Aberrant viruses in cells infected with murine sarcoma virus-feline leukemia virus. J Natl Cancer Inst. 1975;54:763–766. - PubMed
-
- Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. - PubMed
-
- Arai T, Matsumoto K, Saitoh K, Ui M, Ito T, Murakami M, Kanegae Y, Saito I, Cosset F L, Takeuchi Y, Iba H. A new system for stringent, high-titer vesicular stomatitis virus G protein-pseudotyped retrovirus vector induction by introduction of Cre recombinase into stable prepackaging cell lines. J Virol. 1998;72:1115–1121. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

