Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst
- PMID: 11333877
- PMCID: PMC114901
- DOI: 10.1128/JVI.75.11.4984-4989.2001
Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst
Abstract
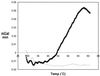
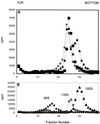
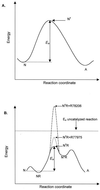
We examined the role of soluble poliovirus receptor on the transition of native poliovirus (160S or N particle) to an infectious intermediate (135S or A particle). The viral receptor behaves as a classic transition state theory catalyst, facilitating the N-to-A conversion by lowering the activation energy for the process by 50 kcal/mol. In contrast to earlier studies which demonstrated that capsid-binding drugs inhibit thermally mediated N-to-A conversion through entropic stabilization alone, capsid-binding drugs are shown to inhibit receptor-mediated N-to-A conversion through a combination of enthalpic and entropic effects.
Figures


References
-
- Caliguiri L A, McSharry J J, Lawrence G W. Effect of arildone on modification of poliovirus in vitro. Virology. 1980;105:86–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

