Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy
- PMID: 11333879
- PMCID: PMC114903
- DOI: 10.1128/JVI.75.11.4999-5008.2001
Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy
Abstract
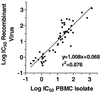
Efavirenz (also known as DMP 266 or SUSTIVA) is a potent nonnucleoside inhibitor of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) activity and of HIV-1 replication in vitro and in vivo. Most patients on efavirenz-containing regimens have sustained antiviral responses; however, rebounds in plasma viral load have been observed in some patients in association with the emergence of mutant strains of HIV-1. Virus isolates from the peripheral blood mononuclear cells (PBMCs) of patients with such treatment failures, as well as recombinant viruses incorporating viral sequences derived from patient plasma, show reduced in vitro susceptibility to efavirenz in association with mutations in the RT gene encoding K103N, Y188L, or G190S/E substitutions. Patterns of RT gene mutations and in vitro susceptibility were similar in plasma virus and in viruses isolated from PBMCs. Variant strains of HIV-1 constructed by site-directed mutagenesis confirmed the role of K103N, G190S, and Y188L substitutions in reduced susceptibility to efavirenz. Further, certain secondary mutations (V106I, V108I, Y181C, Y188H, P225H, and F227L) conferred little resistance to efavirenz as single mutations but enhanced the level of resistance of viruses carrying these mutations in combination with K103N or Y188L. Viruses with K103N or Y188L mutations, regardless of the initial selecting nonnucleoside RT inhibitor (NNRTI), exhibited cross-resistance to all of the presently available NNRTIs (efavirenz, nevirapine, and delavirdine). Some virus isolates from nevirapine or delavirdine treatment failures that lacked K103N or Y188L mutations remained susceptible to efavirenz in vitro, although the clinical significance of this finding is presently unclear.
Figures
References
-
- Bacheler L, Ploughman L, Hertogs K, Larder B. Impact of baseline NNRTI resistance on the efficacy of efavirenz combination therapy in NNRTI therapy-naive patients (Study DMP 266–006) Antivir Ther. 2000;5(Suppl.):70.
-
- Bacheler L T, Anton Elizabeth D, Kudish P, Baker D, Bunville J, Krakowski K, Bolling L, Aujay M, Wang X, Ellis D, Becker M F, Lasut A L, George H J, Spalding D R, Hollis G, Abremski K. Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob Agents Chemother. 2000;44:475–484. - PMC - PubMed
-
- Cheeseman S, Havlir D, McLaughlin M, Greenough T, Sullivan J, Hall D, Hattox S, Spector S, Stein D, Myers M, et al. Phase I/II evaluation of nevirapine alone and in combination with zidovudine for infection with human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:141–151. - PubMed
-
- Demeter L M, Shafer R W, Meehan P M, Holden-Wiltse J, Fischl M A, Freimuth W W, Para M F, Reichman R C. Delavirdine susceptibilities and associated reverse transcriptase mutations in human immunodeficiency virus type 1 isolates from patients in a phase I/II trial of delavirdine monotherapy (ACTG 260) Antimicrob Agents Chemother. 2000;44:794–797. - PMC - PubMed
-
- Falloon J, Piscitelli S, Vogel S, Sadler B, Mitsuya H, Kavlick M, Yoshimura K, Rogers M, LaFon S, Manion D, Lane H, Masur H. Combination therapy with amprenavir, abacavir, and efavirenz in human immunodeficiency virus (HIV)-infected patients failing a protease-inhibitor regimen: pharmacokinetic drug interactions and antiviral activity. Clin Infect Dis. 2000;30:313–318. - PubMed
Publication types
MeSH terms
Substances
Associated data
- GDB/AF349318
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

