Rat cytomegalovirus major immediate-early enhancer switching results in altered growth characteristics
- PMID: 11333888
- PMCID: PMC114912
- DOI: 10.1128/JVI.75.11.5076-5083.2001
Rat cytomegalovirus major immediate-early enhancer switching results in altered growth characteristics
Abstract
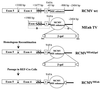
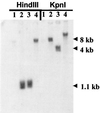
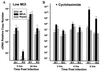
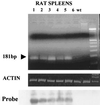
It has been hypothesized that the major immediate-early (MIE) enhancer of cytomegalovirus (CMV) is important in determining virus tropism and latency because of its essential role in initiating the cascade of early gene expression necessary for virus replication. Although rat CMV (RCMV) and murine CMV (MCMV) exhibit extreme species specificity in vivo, they differ in their ability to replicate in tissue culture. MCMV can replicate in a rat embryo fibroblast (REF) cell line while RCMV does not grow in murine fibroblasts. The tropism is not due to a block in virus entry into the cell. We have constructed a recombinant RCMV in which the RCMV MIE enhancer has been replaced with that of MCMV. Growth of the recombinant virus in tissue culture remains restricted to rat cells, suggesting that other viral and/or host factors are more important in determining in vitro tropism. Unlike findings using recombinant MCMV in which the human CMV (HCMV) MIE enhancer substitutes for the native one (A. Angulo, M. Messerle, U. H. Koszinowski, and P. Ghazal, J. Virol. 72:8502-8509, 1998), infection with our recombinant virus at a low multiplicity of infection resulted in a substantial decrease in virus replication. This occurred despite comparable or increased MIE transcription from the recombinant virus. In vivo experiments showed that the recombinant virus replicates normally in the spleen during acute infection. Notably, the recombinant virus appears to be deficient in spreading to the salivary gland, suggesting a role for the MIE enhancer in tropism for certain tissues involved in virus dissemination. Four months after infection, recombinant virus with the foreign MIE enhancer was reactivated from spleen explants.
Figures


Similar articles
-
In vivo replication of recombinant murine cytomegalovirus driven by the paralogous major immediate-early promoter-enhancer of human cytomegalovirus.J Virol. 1999 Jun;73(6):5043-55. doi: 10.1128/JVI.73.6.5043-5055.1999. J Virol. 1999. PMID: 10233967 Free PMC article.
-
The human cytomegalovirus major immediate-early enhancer determines the efficiency of immediate-early gene transcription and viral replication in permissive cells at low multiplicity of infection.J Virol. 2003 Mar;77(6):3602-14. doi: 10.1128/jvi.77.6.3602-3614.2003. J Virol. 2003. PMID: 12610136 Free PMC article.
-
An essential role of the enhancer for murine cytomegalovirus in vivo growth and pathogenesis.J Virol. 2003 Mar;77(5):3217-28. doi: 10.1128/jvi.77.5.3217-3228.2003. J Virol. 2003. PMID: 12584345 Free PMC article.
-
Role of the cytomegalovirus major immediate early enhancer in acute infection and reactivation from latency.Med Microbiol Immunol. 2008 Jun;197(2):223-31. doi: 10.1007/s00430-007-0069-7. Epub 2007 Dec 19. Med Microbiol Immunol. 2008. PMID: 18097687 Review.
-
Mechanisms of human cytomegalovirus persistence and latency.Front Biosci. 2002 Jun 1;7:d1575-82. doi: 10.2741/jarvis. Front Biosci. 2002. PMID: 12045013 Review.
Cited by
-
Enhancerless cytomegalovirus is capable of establishing a low-level maintenance infection in severely immunodeficient host tissues but fails in exponential growth.J Virol. 2010 Jun;84(12):6254-61. doi: 10.1128/JVI.00419-10. Epub 2010 Apr 7. J Virol. 2010. PMID: 20375164 Free PMC article.
-
Cytomegalovirus in Ulcerative Colitis: An Unwanted "Guest".Pathogens. 2024 Aug 2;13(8):650. doi: 10.3390/pathogens13080650. Pathogens. 2024. PMID: 39204250 Free PMC article. Review.
-
Cytomegalovirus e127 protein interacts with the inhibitory CD200 receptor.J Virol. 2011 Jun;85(12):6055-9. doi: 10.1128/JVI.00064-11. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471232 Free PMC article.
-
A Review of Murine Cytomegalovirus as a Model for Human Cytomegalovirus Disease-Do Mice Lie?Int J Mol Sci. 2020 Dec 28;22(1):214. doi: 10.3390/ijms22010214. Int J Mol Sci. 2020. PMID: 33379272 Free PMC article. Review.
-
Requirement of multiple cis-acting elements in the human cytomegalovirus major immediate-early distal enhancer for viral gene expression and replication.J Virol. 2002 Jan;76(1):313-26. doi: 10.1128/jvi.76.1.313-326.2002. J Virol. 2002. PMID: 11739696 Free PMC article.
References
-
- Angulo A, Ghazal P. Regulation of human cytomegalovirus by retinoic acid. Scand J Infect Dis. 1995;99:113–115. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

