Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection
- PMID: 11333895
- PMCID: PMC114919
- DOI: 10.1128/JVI.75.11.5141-5150.2001
Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection
Abstract
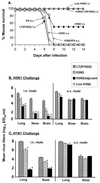
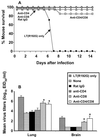
Influenza vaccines that induce greater cross-reactive or heterosubtypic immunity (Het-I) may overcome limitations in vaccine efficacy imposed by the antigenic variability of influenza A viruses. We have compared mucosal versus traditional parenteral administration of inactivated influenza vaccine for the ability to induce Het-I in BALB/c mice and evaluated a modified Escherichia coli heat-labile enterotoxin adjuvant, LT(R192G), for augmentation of Het-I. Mice that received three intranasal (i.n.) immunizations of H3N2 vaccine in the presence of LT(R192G) were completely protected against lethal challenge with a highly pathogenic human H5N1 virus and had nasal and lung viral titers that were at least 2,500-fold lower than those of control mice receiving LT(R192G) alone. In contrast, mice that received three vaccinations of H3N2 vaccine subcutaneously in the presence or absence of LT(R192G) or incomplete Freund's adjuvant were not protected against lethal challenge and had no significant reductions in tissue virus titers observed on day 5 post-H5N1 virus challenge. Mice that were i.n. administered H3N2 vaccine alone, without LT(R192G), displayed partial protection against heterosubtypic challenge. The immune mediators of Het-I were investigated. The functional role of B and CD8+ T cells in Het-I were evaluated by using gene-targeted B-cell (IgH-6(-/-))- or beta2-microglobulin (beta2m(-/-))-deficient mice, respectively. beta2m(-/-) but not IgH-6(-/-) vaccinated mice were protected by Het-I and survived a lethal infection with H5N1, suggesting that B cells, but not CD8+ T cells, were vital for protection of mice against heterosubtypic challenge. Nevertheless, CD8+ T cells contributed to viral clearance in the lungs and brain tissues of heterotypically immune mice. Mucosal but not parenteral vaccination induced subtype cross-reactive lung immunoglobulin G (IgG), IgA, and serum IgG anti-hemagglutinin antibodies, suggesting the presence of a common cross-reactive epitope in the hemagglutinins of H3 and H5. These results suggest a strategy of mucosal vaccination that stimulates cross-protection against multiple influenza virus subtypes, including viruses with pandemic potential.
Figures
Similar articles
-
Protective immunity against influenza H5N1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant.Virology. 2008 Oct 25;380(2):412-20. doi: 10.1016/j.virol.2008.08.002. Epub 2008 Sep 10. Virology. 2008. PMID: 18786689
-
Intranasal immunization with live recombinant Lactococcus lactis combined with heat-labile toxin B subunit protects chickens from highly pathogenic avian influenza H5N1 virus.J Med Virol. 2015 Jan;87(1):39-44. doi: 10.1002/jmv.23983. Epub 2014 May 26. J Med Virol. 2015. PMID: 24861477
-
Heterosubtypic cross-protection induced by whole inactivated influenza virus vaccine in mice: influence of the route of vaccine administration.Influenza Other Respir Viruses. 2013 Nov;7(6):1202-9. doi: 10.1111/irv.12142. Epub 2013 Sep 16. Influenza Other Respir Viruses. 2013. PMID: 24102979 Free PMC article.
-
A proposal for safety standards for human use of cholera toxin (or Escherichia coli heat-labile enterotoxin) derivatives as an adjuvant of nasal inactivated influenza vaccine.Jpn J Infect Dis. 2000 Jun;53(3):98-106. Jpn J Infect Dis. 2000. PMID: 10957706 Review.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
Cited by
-
A novel bivalent vaccine based on a PB2-knockout influenza virus protects mice from pandemic H1N1 and highly pathogenic H5N1 virus challenges.J Virol. 2013 Jul;87(14):7874-81. doi: 10.1128/JVI.00076-13. Epub 2013 May 8. J Virol. 2013. PMID: 23658445 Free PMC article.
-
Oral clarithromycin enhances airway immunoglobulin A (IgA) immunity through induction of IgA class switching recombination and B-cell-activating factor of the tumor necrosis factor family molecule on mucosal dendritic cells in mice infected with influenza A virus.J Virol. 2012 Oct;86(20):10924-34. doi: 10.1128/JVI.01207-12. Epub 2012 Aug 15. J Virol. 2012. PMID: 22896605 Free PMC article.
-
Cross-protective immune responses elicited by live attenuated influenza vaccines.Yonsei Med J. 2013 Mar 1;54(2):271-82. doi: 10.3349/ymj.2013.54.2.271. Yonsei Med J. 2013. PMID: 23364956 Free PMC article. Review.
-
Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus.J Virol. 2007 Apr;81(7):3514-24. doi: 10.1128/JVI.02052-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251294 Free PMC article.
-
Genetic fusions of heat-labile toxoid (LT) and heat-stable toxin b (STb) of porcine enterotoxigenic Escherichia coli elicit protective anti-LT and anti-STb antibodies.Clin Vaccine Immunol. 2010 Aug;17(8):1223-31. doi: 10.1128/CVI.00095-10. Epub 2010 May 26. Clin Vaccine Immunol. 2010. PMID: 20505006 Free PMC article.
References
-
- Ada G L, Jones P D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. - PubMed
-
- Armerding D, Rossiter H, Ghazzouli I, Liehl E. Evaluation of live and inactivated influenza A virus vaccines in a mouse model. J Infect Dis. 1982;145:320–330. - PubMed
-
- Belshe R B, Gruber W C, Mendelman P M, Cho I C, Reisinger K, Block S L, Wittles J, Iacuzio D, Piedra P, Treanor J, King J, Kotloff K, Bernstein D I, Hayden F G, Zangwill K, Yan L, Wolff M. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–175. - PubMed
-
- Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, Gregory V, Cameron K, Lim W, Subbarao K. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997–1998. Virology. 1999;254:115–123. - PubMed
-
- Bukowski J F, Morita C T, Brenner M B. Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol. 1994;153:5133–5140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

