Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination
- PMID: 11333896
- PMCID: PMC114920
- DOI: 10.1128/JVI.75.11.5151-5158.2001
Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination
Abstract
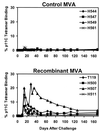
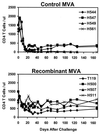
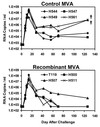
Since cytotoxic T lymphocytes (CTLs) are critical for controlling human immunodeficiency virus type 1 (HIV-1) replication in infected individuals, candidate HIV-1 vaccines should elicit virus-specific CTL responses. In this report, we study the immune responses elicited in rhesus monkeys by a recombinant poxvirus vaccine and the degree of protection afforded against a pathogenic simian-human immunodeficiency virus SHIV-89.6P challenge. Immunization with recombinant modified vaccinia virus Ankara (MVA) vectors expressing SIVmac239 gag-pol and HIV-1 89.6 env elicited potent Gag-specific CTL responses but no detectable SHIV-specific neutralizing antibody (NAb) responses. Following intravenous SHIV-89.6P challenge, sham-vaccinated monkeys developed low-frequency CTL responses, low-titer NAb responses, rapid loss of CD4+ T lymphocytes, high-setpoint viral RNA levels, and significant clinical disease progression and death in half of the animals by day 168 postchallenge. In contrast, the recombinant MVA-vaccinated monkeys demonstrated high-frequency secondary CTL responses, high-titer secondary SHIV-89.6-specific NAb responses, rapid emergence of SHIV-89.6P-specific NAb responses, partial preservation of CD4+ T lymphocytes, reduced setpoint viral RNA levels, and no evidence of clinical disease or mortality by day 168 postchallenge. There was a statistically significant correlation between levels of vaccine-elicited CTL responses prior to challenge and the control of viremia following challenge. These results demonstrate that immune responses elicited by live recombinant vectors, although unable to provide sterilizing immunity, can control viremia and prevent disease progression following a highly pathogenic AIDS virus challenge.
Figures



References
-
- Allen T M, Sidney J, del Guercio M-F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J Immunol. 1998;160:6062–6071. - PubMed
-
- Allen T M, Vogel T U, Fuller D H, Mothe B R, Steffen S, Boyson J E, Shipley T, Fuller J, Hanke T, Sette A, Altman J D, Moss B, McMichael A J, Watkins D I. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000;164:4968–4978. - PubMed
-
- Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
-
- Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. - PubMed
-
- Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiple deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

