Polyomavirus small t antigen prevents retinoic acid-induced retinoblastoma protein hypophosphorylation and redirects retinoic acid-induced G0 arrest and differentiation to apoptosis
- PMID: 11333911
- PMCID: PMC114935
- DOI: 10.1128/JVI.75.11.5302-5314.2001
Polyomavirus small t antigen prevents retinoic acid-induced retinoblastoma protein hypophosphorylation and redirects retinoic acid-induced G0 arrest and differentiation to apoptosis
Abstract
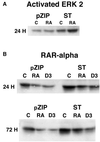
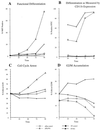
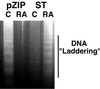
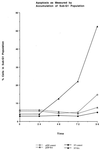
Polyomavirus small t antigen (ST) impedes late features of retinoic acid (RA)-induced HL-60 myeloid differentiation as well as growth arrest, causing apoptosis instead. HL-60 cells were stably transfected with ST. ST slowed the cell cycle, retarding G2/M in particular. Treated with RA, the ST transfectants continued to proliferate and underwent apoptosis. ST also impeded the normally RA-induced hypophosphorylation of the retinoblastoma tumor suppressor protein consistent with failure of the cells to arrest growth. The RA-treated transfectants expressed CD11b, an early cell surface differentiation marker, but inducible oxidative metabolism, a later and more mature functional differentiation marker, was largely inhibited. Instead, the cells underwent apoptosis. ST affected significant known components of RA signaling that result in G0 growth arrest and differentiation in wild-type HL-60. ST increased the basal amount of activated ERK2, which normally increases when wild-type cells are treated with RA. ST caused increased RARalpha expression, which is normally down regulated in RA-treated wild-type cells. The effects of ST on RA-induced myeloid differentiation did not extend to monocytic differentiation and G0 arrest induced by 1,25-dihydroxy vitamin D3, whose receptor is also a member of the steroid-thyroid hormone superfamily. In this case, ST abolished the usually induced G0 arrest and retarded, but did not block, differentiation without inducing apoptosis, thus uncoupling growth arrest and differentiation. In sum, the data show that ST disrupted the normal RA-induced program of G0 arrest and differentiation, causing the cells to abort differentiation and undergo apoptosis.
Figures





Similar articles
-
Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest.Cancer Res. 1998 Jul 15;58(14):3163-72. Cancer Res. 1998. PMID: 9679985
-
Transformation-defective polyoma middle T antigen mutants defective in PLCgamma, PI-3, or src kinase activation enhance ERK2 activation and promote retinoic acid-induced, cell differentiation like wild-type middle T.Exp Cell Res. 1999 May 1;248(2):538-51. doi: 10.1006/excr.1999.4423. Exp Cell Res. 1999. PMID: 10222145
-
Increasing c-FMS (CSF-1 receptor) expression decreases retinoic acid concentration needed to cause cell differentiation and retinoblastoma protein hypophosphorylation.Cancer Res. 1997 May 15;57(10):2020-8. Cancer Res. 1997. PMID: 9158000
-
Post-translational modification of retinoic acid receptor alpha and its roles in tumor cell differentiation.Biochem Pharmacol. 2020 Jan;171:113696. doi: 10.1016/j.bcp.2019.113696. Epub 2019 Nov 11. Biochem Pharmacol. 2020. PMID: 31726045 Review.
-
Retinoic acid-induced cell cycle arrest of human myeloid cell lines.Leuk Lymphoma. 2003 Oct;44(10):1641-50. doi: 10.1080/1042819031000083316. Leuk Lymphoma. 2003. PMID: 14692514 Review.
Cited by
-
Nicotinamide cooperates with retinoic acid and 1,25-dihydroxyvitamin D(3) to regulate cell differentiation and cell cycle arrest of human myeloblastic leukemia cells.Oncology. 2009;76(2):91-100. doi: 10.1159/000188664. Epub 2009 Jan 6. Oncology. 2009. PMID: 19127080 Free PMC article.
-
A novel retinoic acid-responsive element regulates retinoic acid-induced BLR1 expression.Mol Cell Biol. 2004 Mar;24(6):2423-43. doi: 10.1128/MCB.24.6.2423-2443.2004. Mol Cell Biol. 2004. PMID: 14993281 Free PMC article.
-
Induction of p53-independent apoptosis by simian virus 40 small t antigen.J Virol. 2001 Oct;75(19):9142-55. doi: 10.1128/JVI.75.19.9142-9155.2001. J Virol. 2001. PMID: 11533178 Free PMC article.
-
Protein phosphatase 2A isoforms utilizing Aβ scaffolds regulate differentiation through control of Akt protein.J Biol Chem. 2013 Nov 1;288(44):32064-73. doi: 10.1074/jbc.M113.497644. Epub 2013 Sep 19. J Biol Chem. 2013. PMID: 24052256 Free PMC article.
-
Comparisons between murine polyomavirus and Simian virus 40 show significant differences in small T antigen function.J Virol. 2011 Oct;85(20):10649-58. doi: 10.1128/JVI.05034-11. Epub 2011 Aug 10. J Virol. 2011. PMID: 21835797 Free PMC article.
References
-
- Battle T, Levine R A, Yen A. Retinoic acid-induced blr1 expression promotes ERK2 activation and cell differentiation in HL-60 cells. Exp Cell Res. 2000;254:287–298. - PubMed
-
- Battle T E, Roberson M S, Zhang T, Varvayanis S, Yen A. Retinoic acid-induced blr1 expression requires RARα, RXR, and MAPK activation and uses ERK2 but not JNK/SAPK to accelerate cell differentiation. Eur J Cell Biol. 2000;80:59–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

