Viral and cellular factors that target the promyelocytic leukemia oncogenic domains strongly activate a glucocorticoid-responsive promoter
- PMID: 11333923
- PMCID: PMC114947
- DOI: 10.1128/JVI.75.11.5391-5397.2001
Viral and cellular factors that target the promyelocytic leukemia oncogenic domains strongly activate a glucocorticoid-responsive promoter
Abstract
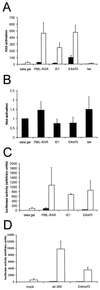
Promyelocytic leukemia (PML) oncogenic domains (PODs) accumulate the transcriptional cofactor named CREB binding protein (CBP) and have been suggested to function as centers of transcription. Transcriptional activation by nuclear hormones, such as glucocorticoids, is augmented by the key constituent of PODs, the PML protein, and decreased by the POD-associated Tax protein of human T-cell leukemia virus type 1 (HTLV-1). This led to the hypothesis that intact PODs might play a positive role in the activation of these promoters. We report here that transiently expressed E4orf3 protein of adenovirus type 5, immediate-early protein 1 of human cytomegalovirus, and the PML-retinoic acid receptor fusion protein from leukemia cells each redistribute CBP within the nucleus. However, unlike the Tax protein of HTLV-1, these factors did not inhibit a glucocorticoid-inducible promoter but strongly enhanced its activity. Thus, at least glucocorticoid-induced transcription does not depend on POD integrity.
Figures



Similar articles
-
The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10).J Virol. 1999 Dec;73(12):10458-71. doi: 10.1128/JVI.73.12.10458-10471.1999. J Virol. 1999. PMID: 10559364 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML.Mol Cell Biol. 1998 Aug;18(8):4899-913. doi: 10.1128/MCB.18.8.4899. Mol Cell Biol. 1998. PMID: 9671498 Free PMC article.
-
Nuclear dots: actors on many stages.Immunobiology. 1997 Dec;198(1-3):307-31. doi: 10.1016/S0171-2985(97)80051-4. Immunobiology. 1997. PMID: 9442402 Review.
-
PML and the oncogenic nuclear domains in regulating transcriptional repression.Curr Opin Cell Biol. 2000 Oct;12(5):641-4. doi: 10.1016/s0955-0674(00)00144-7. Curr Opin Cell Biol. 2000. PMID: 10978902 Review.
Cited by
-
Virion factors that target Daxx to overcome intrinsic immunity.J Virol. 2013 Oct;87(19):10412-22. doi: 10.1128/JVI.00425-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864634 Free PMC article. Review.
-
Diverse roles for E4orf3 at late times of infection revealed in an E1B 55-kilodalton protein mutant background.J Virol. 2004 Sep;78(18):9924-35. doi: 10.1128/JVI.78.18.9924-9935.2004. J Virol. 2004. PMID: 15331726 Free PMC article.
-
Mutual interference of adenovirus infection and myc expression.J Virol. 2003 Jul;77(14):7936-44. doi: 10.1128/jvi.77.14.7936-7944.2003. J Virol. 2003. PMID: 12829833 Free PMC article.
-
Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells.J Virol. 2004 Jun;78(12):6527-42. doi: 10.1128/JVI.78.12.6527-6542.2004. J Virol. 2004. PMID: 15163746 Free PMC article.
-
The adenovirus E4 ORF3 protein binds and reorganizes the TRIM family member transcriptional intermediary factor 1 alpha.J Virol. 2007 Apr;81(8):4264-71. doi: 10.1128/JVI.02629-06. Epub 2007 Feb 7. J Virol. 2007. PMID: 17287283 Free PMC article.
References
-
- Angulo A, Ghazal P. Regulation of human cytomegalovirus by retinoic acid. Scand J Infect Dis Suppl. 1995;99:113–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

