Increased osteoblast apoptosis in apert craniosynostosis: role of protein kinase C and interleukin-1
- PMID: 11337381
- PMCID: PMC1891948
- DOI: 10.1016/S0002-9440(10)64139-9
Increased osteoblast apoptosis in apert craniosynostosis: role of protein kinase C and interleukin-1
Abstract
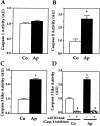
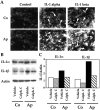
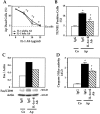
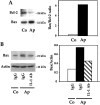
Apert syndrome is an autosomal dominant disorder characterized by premature cranial ossification resulting from fibroblast growth factor receptor-2 (FGFR-2)-activating mutations. We have studied the effects of the prominent S252W FGFR-2 Apert mutation on apoptosis and the underlying mechanisms in human mutant osteoblasts. In vivo analysis of terminal deoxynucleotidyl transferase-mediated nick-end labeling revealed premature apoptosis of mature osteoblasts and osteocytes in the Apert suture compared to normal coronal suture. In vitro, mutant osteoblasts showed increased apoptosis, as demonstrated by terminal deoxynucleotidyl transferase-mediated nick-end labeling analysis, trypan blue staining, and DNA fragmentation. Mutant osteoblasts also showed increased activity of caspase-8 and effector caspases (-3, -6, -7) constitutively. This was related to protein kinase C activation because the selective protein kinase C inhibitor calphostin C inhibited caspase-8, effector caspases, and apoptosis in mutant osteoblasts. Apert osteoblasts also showed increased expression of interleukin (IL)-1alpha, IL-1beta, Fas, and Bax, and decreased Bcl-2 levels. Specific neutralizing anti-IL-1 antibody reduced Fas levels, Bax expression, effector caspases activity, and apoptosis in mutant cells. Thus, the Apert S252W FGFR-2 mutation promotes apoptosis in human osteoblasts through activation of protein kinase C, overexpression of IL-1 and Fas, activation of caspase-8, and increased Bax/Bcl-2 levels, leading to increased effector caspases and DNA fragmentation. This identifies a complex FGFR-2 signaling pathway involved in the premature apoptosis induced by the Apert S252W FGFR-2 mutation in human calvaria osteoblasts.
Figures

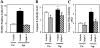
References
-
- Muenke M, Schell U: Fibroblast-growth-factor receptor mutations in human skeletal disorders. Trends Genet 1995, 11:308-313 - PubMed
-
- Naski MC, Ornitz DM: FGF signaling in skeletal development. Front Biosci 1998, 3:D781-D794 - PubMed
-
- Burke D, Wilkes D, Blundell TL, Malcolm S: Fibroblast growth factor receptors: lessons from the genes. Trends Biochem Sci 1998, 23:59-62 - PubMed
-
- Hehr U, Muenke M: Craniosynostosis syndromes: from genes to premature fusion of skull bones. Mol Genet Metab 1999, 68:139-151 - PubMed
-
- Mangasarian K, Li Y, Mansukhani A, Basilico C: Mutation associated with Crouzon syndrome causes ligand-independent dimerization and activation of FGF receptor-2. J Cell Physiol 1997, 172:117-125 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

