Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein
- PMID: 11337384
- PMCID: PMC1891942
- DOI: 10.1016/S0002-9440(10)64142-9
Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein
Abstract
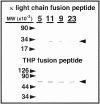
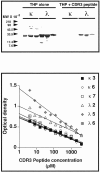
Cast nephropathy, or myeloma kidney, is a potentially reversible cause of chronic renal failure. In this condition, filtered light chains bind to a common site on Tamm-Horsfall protein (THP), which is produced by cells of the thick ascending limb of the loop of HENLE: Subsequent aggregation of these proteins produces casts that obstruct tubule fluid flow and results in renal failure. In the present study, we used the yeast two-hybrid system to determine the site of interaction of light chains with THP. The third complementarity-determining region (CDR3) of both kappa and lambda light chains interacted with THP. These findings were confirmed in a series of competition studies using a synthetic peptide that corresponded to the CDR3 region and purified THP and light chains. Variations in the CDR3 sequence of the light chain affected binding. Thus, the current studies increase our understanding of the process of cast formation and provide an opportunity to develop strategies that may inhibit this interaction and prevent the clinical manifestations of myeloma kidney.
Figures


Similar articles
-
Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation.J Clin Invest. 1993 Dec;92(6):2975-83. doi: 10.1172/JCI116920. J Clin Invest. 1993. PMID: 8254051 Free PMC article.
-
Localization of a single binding site for immunoglobulin light chains on human Tamm-Horsfall glycoprotein.J Clin Invest. 1997 Feb 15;99(4):732-6. doi: 10.1172/JCI119218. J Clin Invest. 1997. PMID: 9045877 Free PMC article.
-
Biochemical interaction between Tamm-Horsfall glycoprotein and Ig light chains in the pathogenesis of cast nephropathy.Lab Invest. 1995 Dec;73(6):810-7. Lab Invest. 1995. PMID: 8558842
-
Pathogenesis and treatment of myeloma kidney.J Lab Clin Med. 1994 Oct;124(4):484-8. J Lab Clin Med. 1994. PMID: 7930873 Review.
-
[Tamm-Horsfall protein].Nephrologie. 1992;13(1):7-11. Nephrologie. 1992. PMID: 1579196 Review. French.
Cited by
-
Pathogenesis of renal failure in multiple myeloma: any role of contrast media?Biomed Res Int. 2014;2014:167125. doi: 10.1155/2014/167125. Epub 2014 Apr 30. Biomed Res Int. 2014. PMID: 24877060 Free PMC article. Review.
-
Lenalidomide and dexamethasone for acute light chain-induced renal failure: a phase II study.Haematologica. 2015 Mar;100(3):385-91. doi: 10.3324/haematol.2014.115204. Epub 2014 Nov 14. Haematologica. 2015. PMID: 25398836 Free PMC article. Clinical Trial.
-
Medullary cystic kidney disease type 1: mutational analysis in 37 genes based on haplotype sharing.Hum Genet. 2006 Jul;119(6):649-58. doi: 10.1007/s00439-006-0176-3. Epub 2006 Apr 26. Hum Genet. 2006. PMID: 16738948
-
Treatment of multiple myeloma with renal involvement: the nephrologist's view.Clin Kidney J. 2018 Dec;11(6):777-785. doi: 10.1093/ckj/sfy065. Epub 2018 Aug 1. Clin Kidney J. 2018. PMID: 30524711 Free PMC article.
-
Myeloma light chain cast nephropathy, a review.J Nephrol. 2019 Apr;32(2):189-198. doi: 10.1007/s40620-018-0492-4. Epub 2018 May 5. J Nephrol. 2019. PMID: 29730782 Review.
References
-
- Jones HB: Papers on chemical pathology: prefaced by the Gulstonian Lectures, read at the Royal College of Physicians, 1846. Lancet 1847, 2:88-92
-
- Baylis C, Falconer-Smith J, Ross B: Glomerular and tubular handling of differently charged human immunoglobulin light chains by the rat kidney. Clin Sci 1988, 74:639-644 - PubMed
-
- Sanders PW, Herrera GA, Galla JH: Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int 1987, 32:851-861 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

