Requirement for p27(KIP1) in retinoblastoma protein-mediated senescence
- PMID: 11340156
- PMCID: PMC86983
- DOI: 10.1128/MCB.21.11.3616-3631.2001
Requirement for p27(KIP1) in retinoblastoma protein-mediated senescence
Abstract
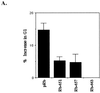
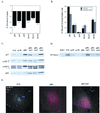
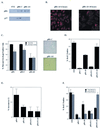
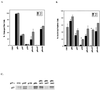
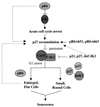
In vivo and in vitro evidence indicate that cells do not divide indefinitely but instead stop growing and undergo a process termed cellular proliferative senescence. Very little is known about how senescence occurs, but there are several indications that the retinoblastoma protein (pRb) is involved, the most striking being that reintroduction of RB into RB(-/-) tumor cell lines induces senescence. In investigating the mechanism by which pRb induces senescence, we have found that pRb causes a posttranscriptional accumulation of the cyclin-dependent kinase inhibitor p27(KIP1) that is accompanied by an increase in p27(KIP1) specifically bound to cyclin E and a concomitant decrease in cyclin E-associated kinase activity. In contrast, pRb-related proteins p107 and p130, which also decrease cyclin E-kinase activity, do not cause an accumulation of p27(KIP1) and induce senescence poorly. In addition, the use of pRb proteins mutated in the pocket domain demonstrates that pRb upregulation of p27(KIP1) and senescence induction do not require the interaction of pRb with E2F. Furthermore, ectopic expression of p21(CIP1) or p27(KIP1) induces senescence but not the morphology change associated with pRb-mediated senescence, uncoupling senescence from the morphological transformation. Finally, the ability of pRb to maintain cell cycle arrest and induce senescence is reversibly abrogated by ablation of p27(KIP1) expression. These findings suggest that prolonged cell cycle arrest through the persistent and specific inhibition of cdk2 activity by p27(KIP1) is critical for pRb-induced senescence.
Figures

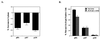
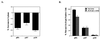
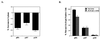


References
-
- Aagaard L, Lukas J, Bartkova J, Kjerulff A A, Strauss M, Bartek J. Aberrations of p16Ink4 and retinoblastoma tumour-suppressor genes occur in distinct sub-sets of human cancer cell lines. Int J Cancer. 1995;61:115–120. - PubMed
-
- Campisi J, Dimiri G, Hara E. Handbook of the biology of aging. 4th ed. New York, N.Y: Academic Press; 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
