Activation of the Ral and phosphatidylinositol 3' kinase signaling pathways by the ras-related protein TC21
- PMID: 11340168
- PMCID: PMC87018
- DOI: 10.1128/MCB.21.11.3750-3762.2001
Activation of the Ral and phosphatidylinositol 3' kinase signaling pathways by the ras-related protein TC21
Abstract
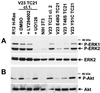
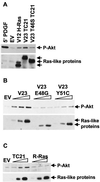
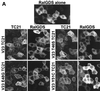
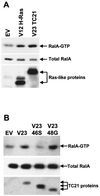
TC21 is a member of the Ras superfamily of small GTP-binding proteins that, like Ras, has been implicated in the regulation of growth-stimulating pathways. We have previously identified the Raf/mitogen-activated protein kinase pathway as a direct TC21 effector pathway required for TC21-induced transformation (M. Rosário, H. F. Paterson, and C. J. Marshall, EMBO J. 18:1270-1279, 1999). In this study we have identified two further effector pathways for TC21, which contribute to TC21-stimulated transformation: the phosphatidylinositol 3' kinase (PI-3K) and Ral signaling pathways. Expression of constitutively active TC21 leads to the activation of Ral A and the PI-3K-dependent activation of Akt/protein kinase B. Strong activation of the PI-3K/Akt pathway is seen even with very low levels of TC21 expression, suggesting that TC21 may be a key small GTPase-regulator of PI-3K. TC21-induced alterations in cellular morphology in NIH 3T3 and PC12 cells are also PI-3K dependent. On the other hand, activation of the Ral pathway by TC21 is required for TC21-stimulated DNA synthesis but not transformed morphology. We show that inhibition of Ral signaling blocks DNA synthesis in human tumor cell lines containing activating mutations in TC21, demonstrating for the first time that this pathway is required for the proliferation of human tumor cells. Finally, we provide mechanisms for the activation of these pathways, namely, the direct in vivo interaction of TC21 with guanine nucleotide exchange factors for Ral, resulting in their translocation to the plasma membrane, and the direct interaction of TC21 with PI-3K. In both cases, the effector domain region of TC21 is required since point mutations in this region can interfere with activation of downstream signaling.
Figures








Similar articles
-
TC21 mediates transformation and cell survival via activation of phosphatidylinositol 3-kinase/Akt and NF-kappaB signaling pathway.Oncogene. 2002 Feb 7;21(7):1062-70. doi: 10.1038/sj.onc.1205154. Oncogene. 2002. PMID: 11850823
-
Signal transduction elements of TC21, an oncogenic member of the R-Ras subfamily of GTP-binding proteins.Oncogene. 1999 Oct 21;18(43):5860-9. doi: 10.1038/sj.onc.1202968. Oncogene. 1999. PMID: 10557073
-
TC21 and Ras share indistinguishable transforming and differentiating activities.Oncogene. 1999 Mar 25;18(12):2107-16. doi: 10.1038/sj.onc.1202517. Oncogene. 1999. PMID: 10321735
-
Ras-induced transformation and signaling pathway.J Biochem. 1999 Nov;126(5):799-803. doi: 10.1093/oxfordjournals.jbchem.a022519. J Biochem. 1999. PMID: 10544270 Review.
-
"New"-clear functions of PDK1: beyond a master kinase in the cytosol?J Cell Biochem. 2005 Dec 15;96(6):1157-62. doi: 10.1002/jcb.20651. J Cell Biochem. 2005. PMID: 16187290 Review.
Cited by
-
RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling.Curr Biol. 2010 Jan 12;20(1):75-9. doi: 10.1016/j.cub.2009.11.016. Epub 2009 Dec 10. Curr Biol. 2010. PMID: 20005108 Free PMC article.
-
The Ras-like protein R-Ras2/TC21 is important for proper mammary gland development.Mol Biol Cell. 2012 Jun;23(12):2373-87. doi: 10.1091/mbc.E12-01-0060. Epub 2012 Apr 25. Mol Biol Cell. 2012. PMID: 22535521 Free PMC article.
-
A Ral guanine exchange factor-Ral pathway is conserved in Drosophila melanogaster and sheds new light on the connectivity of the Ral, Ras, and Rap pathways.Mol Cell Biol. 2003 Feb;23(3):1112-24. doi: 10.1128/MCB.23.3.1112-1124.2003. Mol Cell Biol. 2003. PMID: 12529414 Free PMC article.
-
RalA interacts with ZONAB in a cell density-dependent manner and regulates its transcriptional activity.EMBO J. 2005 Jan 12;24(1):54-62. doi: 10.1038/sj.emboj.7600497. Epub 2004 Dec 9. EMBO J. 2005. PMID: 15592429 Free PMC article.
-
The Expression, Purification, and Characterization of a Ras Oncogene (Bras2) in Silkworm (Bombyx mori).Int J Genomics. 2013;2013:269609. doi: 10.1155/2013/269609. Epub 2013 May 27. Int J Genomics. 2013. PMID: 23781494 Free PMC article.
References
-
- Bos J L. Ras-like GTPases. Biochim Biophys Acta. 1997;1333:M19–M31. - PubMed
-
- Carboni J M, Yan N, Cox A D, Bustelo X R, Graham S M, Lynch M J, Weinmann R, Seizinger B R, Der C J, Barbacid M, Manne V. Farnesyltransferase inhibitors are inhibitors of Ras but not R-Ras2/TC21, transformation. Oncogene. 1995;10:1905–1913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
