Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: implications for the mechanism of protective energy dissipation
- PMID: 11340191
- PMCID: PMC135551
- DOI: 10.1105/tpc.13.5.1193
Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: implications for the mechanism of protective energy dissipation
Abstract
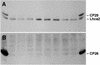
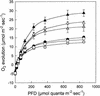
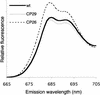
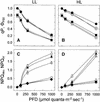
The specific roles of the chlorophyll a/b binding proteins CP29 and CP26 in light harvesting and energy dissipation within the photosynthetic apparatus have been investigated. Arabidopsis was transformed with antisense constructs against the genes encoding the CP29 or CP26 apoprotein, which gave rise to several transgenic lines with remarkably low amounts of the antisense target proteins. The decrease in the level of CP24 protein in the CP29 antisense lines indicates a physical interaction between these complexes. Analysis of chlorophyll fluorescence showed that removal of the proteins affected photosystem II function, probably as a result of changes in the organization of the light-harvesting antenna. However, whole plant measurements showed that overall photosynthetic rates were similar to those in the wild type. Both antisense lines were capable of the qE type of nonphotochemical fluorescence quenching, although there were minor changes in the capacity for quenching and in its induction kinetics. High-light-induced violaxanthin deepoxidation to zeaxanthin was not affected, although the pool size of these pigments was decreased slightly. We conclude that CP29 and CP26 are unlikely to be sites for nonphotochemical quenching.
Figures




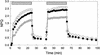
References
-
- Bassi, R., and Dainese, P. (1992). A supramolecular light-harvesting complex from chloroplast photosystem-II membranes. Eur. J. Biochem. 204 317–326. - PubMed
-
- Bassi, R., Giuffra, E., Croce, R., Dainese, P., and Bergantino, E. (1996). Biochemistry and molecular biology of pigment binding proteins. In Light as an Energy Source and Information Carrier in Plant Physiology, R.C. Jennings, G. Zucchelli, F. Ghetti, and G. Colombetti, eds (New York: Plenum Press), pp. 41–63.
-
- Bassi, R., Sandona, D., and Croce, R. (1997). Novel aspects of chlorophyll a/b-binding proteins. Physiol. Plant. 100 769–779.
-
- Boekema, E.J., van Roon, H., and Dekker, J.P. (1998). Specific association of photosystem II and light-harvesting complex II in partially solubilized photosystem II membranes. FEBS Lett. 424 95–99. - PubMed
-
- Britton, G. (1995). UV/visible spectroscopy. In Carotenoids, Vol. 1B, Spectroscopy, G. Britton, S. Liaaen-Jensen, and H. Pfander, eds (Basel, Switzerland: Birkhauser Verlag), pp. 458–488.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

