Short-chain fatty acid derivatives stimulate cell proliferation and induce STAT-5 activation
- PMID: 11342457
- PMCID: PMC4263369
- DOI: 10.1182/blood.v97.10.3259
Short-chain fatty acid derivatives stimulate cell proliferation and induce STAT-5 activation
Abstract
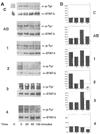
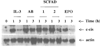
Current chemotherapeutic and butyrate therapeutics that induce fetal hemoglobin expression generally also suppress erythropoiesis, limiting the production of cells containing fetal hemoglobin (F cells). Recently, selected short-chain fatty acid derivatives (SCFADs) were identified that induce endogenous gamma-globin expression in K562 cells and human burst-forming units-erythroid and that increase proliferation of human erythroid progenitors and a multilineage interleukin-3-dependent hematopoietic cell line. In this report, gamma-globin inducibility by these SCFADs was further demonstrated in mice transgenic for the locus control region and the entire beta-globin gene locus in a yeast artificial chromosome and in 2 globin promoter-reporter assays. Conditioned media experiments strongly suggest that their proliferative activity is a direct effect of the test compounds. Investigation of potential mechanisms of action of these SCFADs demonstrates that these compounds induce prolonged expression of the growth-promoting genes c-myb and c-myc. Both butyrate and specific growth-stimulatory SCFADs induced prolonged signal transducer and activator of transcription (STAT)-5 phosphorylation and activation, and c-cis expression, persisting for more than 120 minutes, whereas with IL-3 alone phosphorylation disappeared within minutes. In contrast to butyrate treatment, the growth-stimulating SCFADs did not result in bulk histone H4 hyperacetylation or induction of p21(Waf/Cip), which mediates the suppression of cellular growth by butyrate. These findings suggest that the absence of bulk histone hyperacetylation and p21 induction, but prolonged induction of cis, myb, myc, and STAT-5 activation, contribute to the cellular proliferation induced by selected SCFADs.
Figures





Similar articles
-
Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo.Blood. 2002 Dec 15;100(13):4640-8. doi: 10.1182/blood-2002-02-0353. Epub 2002 Aug 15. Blood. 2002. PMID: 12393583 Free PMC article.
-
Effects of thrombopoietin, interleukin-3 and the kinase inhibitor K-252a on growth and polyploidization of the megakaryocytic cell line M-07e.Leukemia. 1998 Oct;12(10):1603-11. doi: 10.1038/sj.leu.2401170. Leukemia. 1998. PMID: 9766506
-
Enhancement of growth and survival and alterations in Bcl-family proteins in beta-thalassemic erythroid progenitors by novel short-chain fatty acid derivatives.Blood Cells Mol Dis. 2005 Sep-Oct;35(2):217-26. doi: 10.1016/j.bcmd.2005.06.007. Blood Cells Mol Dis. 2005. PMID: 16099182 Free PMC article.
-
Prolactin-induced expression of cytokine-inducible SH2 signaling inhibitors in human hematopoietic progenitors.Exp Hematol. 2001 Aug;29(8):937-42. doi: 10.1016/s0301-472x(01)00673-7. Exp Hematol. 2001. PMID: 11495699
-
Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation.Proc Nutr Soc. 2003 Feb;62(1):101-6. doi: 10.1079/PNS2002215. Proc Nutr Soc. 2003. PMID: 12740064 Review.
Cited by
-
Hydroxyurea responsiveness in β-thalassemic patients is determined by the stress response adaptation of erythroid progenitors and their differentiation propensity.Haematologica. 2013 May;98(5):696-704. doi: 10.3324/haematol.2012.074492. Epub 2012 Oct 25. Haematologica. 2013. PMID: 23100274 Free PMC article.
-
Fetal globin gene inducers: novel agents and new potential.Ann N Y Acad Sci. 2010 Aug;1202:158-64. doi: 10.1111/j.1749-6632.2010.05593.x. Ann N Y Acad Sci. 2010. PMID: 20712788 Free PMC article.
-
A centric view of JAK/STAT5 in intestinal homeostasis, infection, and inflammation.Cytokine. 2021 Mar;139:155392. doi: 10.1016/j.cyto.2020.155392. Epub 2021 Jan 19. Cytokine. 2021. PMID: 33482575 Free PMC article. Review.
-
Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis.Blood. 2007 Oct 15;110(8):2864-71. doi: 10.1182/blood-2007-01-065201. Epub 2007 Jul 9. Blood. 2007. PMID: 17620452 Free PMC article.
-
Histone deacetylase 9 activates gamma-globin gene expression in primary erythroid cells.J Biol Chem. 2011 Jan 21;286(3):2343-53. doi: 10.1074/jbc.M110.115725. Epub 2010 Nov 13. J Biol Chem. 2011. PMID: 21078662 Free PMC article.
References
-
- Burns LJ, Glauber JG, Ginder GD. Butyrate induces selective transcriptional activation of a hypomethylated embryonic globin gene in adult erythroid cells. Blood. 1988;72:1536–1542. - PubMed
-
- Perrine SP, Greene MF, Faller DV. Delay in the fetal globin switch in infants of diabetic mothers. N Engl J Med. 1985;312:334–338. - PubMed
-
- Perrine SP, Miller BA, Faller DV, et al. Sodium butyrate stimulates fetal globin gene expression in erythroid progenitors of children with HbSS and beta thalassemia. Blood. 1989;74:454–460. - PubMed
-
- Constantoulakis P, Knitter G, Stamatoyannopoulos G. On the induction of fetal hemoglobin by butyrates: in vivo and in vitro studies with sodium butyrate and comparison with treatments with 5 azaC and araC. Blood. 1989;74:1963–1971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

