The Ehlers-Danlos syndrome: on beyond collagens
- PMID: 11342567
- PMCID: PMC209288
- DOI: 10.1172/JCI12881
The Ehlers-Danlos syndrome: on beyond collagens
Figures
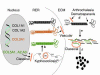

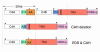
References
-
- Steinmann, B., Royce, P., and Superti-Furga, A. 1993. The Ehlers-Danlos Syndrome. In Connective tissue and its heritable disorders. P. Royce and B. Steinmann, editors. Wiley-Liss. New York, New York, USA. 351–407.
-
- Byers, P. 1995. Disorders of collagen biosynthesis and structure. In The metabolic basis of inherited disease. C. Scriver, A. Beaudet, W. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 4029–4075.
-
- Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77:31–37. - PubMed
-
- Pepin M, Schwarze U, Superti-Furga A, Byers P. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–680. - PubMed

