Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure
- PMID: 11342578
- PMCID: PMC209282
- DOI: 10.1172/JCI12089
Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure
Abstract
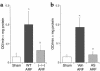
We sought to determine whether mice deficient in the proinflammatory caspase-1, which cleaves precursors of IL-1 beta and IL-18, were protected against ischemic acute renal failure (ARF). Caspase-1(-/-) mice developed less ischemic ARF as judged by renal function and renal histology. These animals had significantly reduced blood urea nitrogen and serum creatinine levels and a lower morphological tubular necrosis score than did wild-type mice with ischemic ARF. Since caspase-1 activates IL-18, lack of mature IL-18 might protect these caspase-1(-/-) mice from ARF. In wild-type animals, we found that ARF causes kidney IL-18 levels to more than double and induces the conversion of the IL-18 precursor to the mature form. This conversion is not observed in caspase-1(-/-) ARF mice or sham-operated controls. We then injected wild-type mice with IL-18-neutralizing antiserum before the ischemic insult and found a similar degree of protection from ARF as seen in caspase-1(-/-) mice. In addition, we observed a fivefold increase in myeloperoxidase activity in control mice with ARF, but no such increase in caspase-1(-/-) or IL-18 antiserum-treated mice. Finally, we confirmed histologically that caspase-1(-/-) mice show decreased neutrophil infiltration, indicating that the deleterious role of IL-18 in ischemic ARF may be due to increased neutrophil infiltration.
Figures

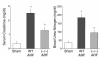

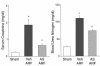

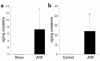
References
-
- Kaushal GP, Ueda N, Shah SV. Role of caspases (ICE/CED 3 proteases) in DNA damage and cell death in response to a mitochondrial inhibitor, antimycin A. Kidney Int. 1997;52:438–445. - PubMed
-
- Edelstein CL, Shi Y, Schrier RW. Role of caspases in hypoxia-induced necrosis of rat renal proximal tubules. J Am Soc Nephrol. 1999;10:1940–1949. - PubMed
-
- Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. - PubMed
-
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

