Heparin-binding defective lipoprotein lipase is unstable and causes abnormalities in lipid delivery to tissues
- PMID: 11342582
- PMCID: PMC209279
- DOI: 10.1172/JCI11774
Heparin-binding defective lipoprotein lipase is unstable and causes abnormalities in lipid delivery to tissues
Abstract
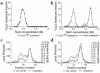
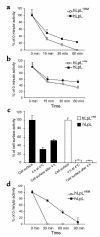
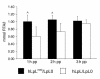
Lipoprotein lipase (LpL) binding to heparan sulfate proteoglycans (HSPGs) is hypothesized to stabilize the enzyme, localize LpL in specific capillary beds, and route lipoprotein lipids to the underlying tissues. To test these hypotheses in vivo, we created mice expressing a human LpL minigene (hLpL(HBM)) carrying a mutated heparin-binding site. Three basic amino acids in the carboxyl terminal region of LpL were mutated, yielding an active enzyme with reduced heparin binding. Mice expressing hLpL(HBM) accumulated inactive human LpL (hLpL) protein in preheparin blood. hLpL(HBM) rapidly lost activity during a 37 degrees C incubation, confirming a requirement for heparin binding to stabilize LPL: Nevertheless, expression of hLpL(HBM) prevented the neonatal demise of LpL knockout mice. On the LpL-deficient background hLpL(HBM) expression led to defective targeting of lipids to tissues. Compared with mice expressing native hLpL in the muscle, hLpL(HBM) transgenic mice had increased postprandial FFAs, decreased lipid uptake in muscle tissue, and increased lipid uptake in kidneys. Thus, heparin association is required for LpL stability and normal physiologic functions. These experiments confirm in vivo that association with HSPGs can provide a means to maintain proteins in their stable conformations and to anchor them at sites where their activity is required.
Figures
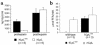



References
-
- Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. - PubMed
-
- Iverius PH, Lindahl U, Egelrud T, Olivecrona T. Effects of heparin on lipoprotein lipase from bovine milk. J Biol Chem. 1972;247:6610–6616. - PubMed
-
- Saxena U, Klein MG, Goldberg IJ. Identification and characterization of the endothelial cell surface lipoprotein lipase receptor. J Biol Chem. 1991;266:17516–17521. - PubMed
-
- Parthasarathy N, et al. Oligosaccharide sequences of endothelial cell surface heparan sulfate proteoglycan with affinity for lipoprotein lipase. J Biol Chem. 1994;269:22391–22396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

