Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia
- PMID: 11342587
- PMCID: PMC2193432
- DOI: 10.1084/jem.193.9.1027
Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia
Abstract
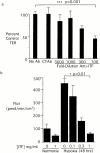
Mucosal organs such as the intestine are supported by a rich and complex underlying vasculature. For this reason, the intestine, and particularly barrier-protective epithelial cells, are susceptible to damage related to diminished blood flow and concomitant tissue hypoxia. We sought to identify compensatory mechanisms that protect epithelial barrier during episodes of intestinal hypoxia. Initial studies examining T84 colonic epithelial cells revealed that barrier function is uniquely resistant to changes elicited by hypoxia. A search for intestinal-specific, barrier-protective factors revealed that the human intestinal trefoil factor (ITF) gene promoter bears a previously unappreciated binding site for hypoxia-inducible factor (HIF)-1. Hypoxia resulted in parallel induction of ITF mRNA and protein. Electrophoretic mobility shift assay analysis using ITF-specific, HIF-1 consensus motifs resulted in a hypoxia-inducible DNA binding activity, and loading cells with antisense oligonucleotides directed against the alpha chain of HIF-1 resulted in a loss of ITF hypoxia inducibility. Moreover, addition of anti-ITF antibody resulted in a loss of barrier function in epithelial cells exposed to hypoxia, and the addition of recombinant human ITF to vascular endothelial cells partially protected endothelial cells from hypoxia-elicited barrier disruption. Extensions of these studies in vivo revealed prominent hypoxia-elicited increases in intestinal permeability in ITF null mice. HIF-1-dependent induction of ITF may provide an adaptive link for maintenance of barrier function during hypoxia.
Figures

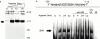


References
-
- Madara J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 1998;60:143–159. - PubMed
-
- Taylor C.T., Colgan S.P. Therapeutic targets for hypoxia-elicited pathways. Pharmacol. Res. 1999;16:1498–1505. - PubMed
-
- Semenza G.L. Perspectives on oxygen sensing. Cell. 1999;98:281–284. - PubMed
-
- Semenza G.L. Hypoxia-inducible factor 1master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998;8:588–594. - PubMed
-
- Wang G.L., Semenza G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995;270:1230–1237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

