The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18)
- PMID: 11342588
- PMCID: PMC2193436
- DOI: 10.1084/jem.193.9.1035
The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18)
Abstract
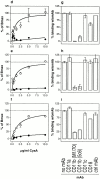
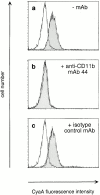
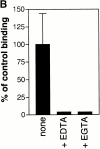
The adenylate cyclase toxin (CyaA) of Bordetella pertussis is a major virulence factor required for the early phases of lung colonization. It can invade eukaryotic cells where, upon activation by endogenous calmodulin, it catalyzes the formation of unregulated cAMP levels. CyaA intoxication leads to evident toxic effects on macrophages and neutrophils. Here, we demonstrate that CyaA uses the alpha(M)beta(2) integrin (CD11b/CD18) as a cell receptor. Indeed, the saturable binding of CyaA to the surface of various hematopoietic cell lines correlated with the presence of the alpha(M)beta(2) integrin on these cells. Moreover, binding of CyaA to various murine cell lines and human neutrophils was specifically blocked by anti-CD11b monoclonal antibodies. The increase of intracellular cAMP level and cell death triggered by CyaA intoxication was also specifically blocked by anti-CD11b monoclonal antibodies. In addition, CyaA bound efficiently and triggered intracellular cAMP increase and cell death in Chinese hamster ovary cells transfected with alpha(M)beta(2) (CD11b/CD18) but not in cells transfected with the vector alone or with the alpha(X)beta(2) (CD11c/CD18) integrin. Thus, the cellular distribution of CD11b, mostly on neutrophils, macrophages, and dendritic and natural killer cells, supports a role for CyaA in disrupting the early, innate antibacterial immune response.
Figures












References
-
- Weiss A.A., Hewlett E.L., Myers G.A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis . J. Infect. Dis. 1984;150:219–222. - PubMed
-
- Khelef N., Sakamoto H., Guiso N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb. Pathog. 1992;12:227–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

