Twofold reduction of phosphofructokinase activity in Lactococcus lactis results in strong decreases in growth rate and in glycolytic flux
- PMID: 11344154
- PMCID: PMC99644
- DOI: 10.1128/JB.183.11.3458-3467.2001
Twofold reduction of phosphofructokinase activity in Lactococcus lactis results in strong decreases in growth rate and in glycolytic flux
Abstract
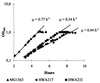
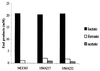
Two mutant strains of Lactococcus lactis in which the promoter of the las operon, harboring pfk, pyk, and ldh, were replaced by synthetic promoters were constructed. These las mutants had an approximately twofold decrease in the activity of phosphofructokinase, whereas the activities of pyruvate kinase and lactate dehydrogenase remained closer to the wild-type level. In defined medium supplemented with glucose, the growth rate of the mutants was reduced to 57 to 70% of wild-type levels and the glycolytic flux was reduced to 62 to 76% of wild-type levels. In complex medium growth was even further reduced. Surprisingly, the mutants still showed homolactic fermentation, which indicated that the limitation was different from standard glucose-limited conditions. One explanation could be that the reduced activity of phosphofructokinase resulted in the accumulation of sugar-phosphates. Indeed, when one of the mutants was starved for glucose in glucose-limited chemostat, the growth rate could gradually be increased to 195% of the growth rate observed in glucose-saturated batch culture, suggesting that phosphofructokinase does affect the concentration of upstream metabolites. The pools of glucose-6-phosphate and fructose-6-phosphate were subsequently found to be increased two- to fourfold in the las mutants, which indicates that phosphofructokinase exerts strong control over the concentration of these metabolites.
Figures


References
-
- Andersen H W. Ph.D. thesis. Regulation and control of the glycolytic enzymes phosphofructokinase and lactate dehydrogenase in Lactococcus lactis. Technical University of DenmarkLyngby, Denmark: Department of Microbiology; 2000.
-
- Bolotin A, Mauger S, Malarme K, Ehrlich S D, Sorokin A. Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Leeuwenhoek. 1999;76:27–76. - PubMed
-
- Crow V L, Pritchard G G. Pyruvate kinase from Streptococcus lactis. Methods Enzymol. 1982;90:165–170. - PubMed
-
- Davidson B E, Llanos R M, Cancilla M R, Redman N C, Hillier A J. Current research on the genetics of lactic acid production in lactic acid bacteria. Int Dairy. 1995;5:763–784.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

