Evolutionary relationships among Rel domains indicate functional diversification by recombination
- PMID: 11344309
- PMCID: PMC33283
- DOI: 10.1073/pnas.101602398
Evolutionary relationships among Rel domains indicate functional diversification by recombination
Abstract
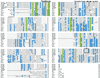
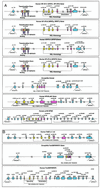
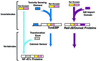
The recent sequencing of several complete genomes has made it possible to track the evolution of large gene families by their genomic structure. Following the large-scale association of exons encoding domains with well defined functions in invertebrates could be useful in predicting the function of complex multidomain proteins in mammals produced by accretion of domains. With this objective, we have determined the genomic structure of the 14 genes in invertebrates and vertebrates that contain rel domains. The sequence encoding the rel domain is defined by intronic boundaries and has been recombined with at least three structurally and functionally distinct genomic sequences to generate coding sequences for: (i) the rel/Dorsal/NFkappaB proteins that are retained in the cytoplasm by IkB-like proteins; (ii) the NFATc proteins that sense calcium signals and undergo cytoplasmic-to-nuclear translocation in response to dephosphorylation by calcineurin; and (iii) the TonEBP tonicity-responsive proteins. Remarkably, a single exon in each NFATc family member encodes the entire Ca(2+)/calcineurin sensing region, including nuclear import/export, calcineurin-binding, and substrate regions. The Rel/Dorsal proteins and the TonEBP proteins are present in Drosophila but not Caenorhabditis elegans. On the other hand, the calcium-responsive NFATc proteins are present only in vertebrates, suggesting that the NFATc family is dedicated to functions specific to vertebrates such as a recombinational immune response, cardiovascular development, and vertebrate-specific aspects of the development and function of the nervous system.
Figures
References
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

