CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited
- PMID: 11349042
- PMCID: PMC98389
- DOI: 10.1128/IAI.69.6.3772-3781.2001
CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited
Erratum in
- Infect Immun 2001 Aug;69(6):5216
Abstract
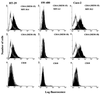
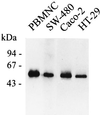
Human endothelial as well as epithelial cells were shown to respond to lipopolysaccharides (LPSs). However, the expression and release of CD14 by these so-called CD14-negative cells have not been studied in detail. We investigated three human intestinal epithelial cell lines (ECLs), SW-480, HT-29, and Caco-2, for their expression of CD14 and CD11c/CD18 as well as their responsiveness to endotoxins. Fluorescence-activated cell sorter analysis revealed no expression of CD11c/CD18, but there was low expression of membrane-bound CD14 on HT-29, Caco-2, and SW-480 ECLs. Both Western blotting and reverse transcription-PCR confirmed the CD14 positivity of all three intestinal ECLs. No substantial modulation of CD14 expression was achieved after 6, 8, 18, 24, and 48 h of cultivation with 10-fold serial dilutions of LPS ranging from 0.01 ng/ml to 100 microg/ml. Interestingly, soluble CD14 was found in the tissue culture supernatants of all three ECLs. Finally, only HT-29 and SW-480, and not Caco-2, cells responded to LPS exposure (range, 0.01 ng/ml to 100 microg/ml) by interleukin 8 release. Thus, we show that HT-29, SW-480, and Caco-2 human intestinal ECLs express membrane-bound CD14. As Caco-2 cells did not respond to LPS, these cell lines might be an interesting model for studying the receptor complex for LPS. The fact that human intestinal epithelial cells are capable not only of expression but also of release of soluble CD14 may have important implications in vivo, e.g., in shaping the interaction between the mucosal immune system and bacteria in the gut and/or in the pathogenesis of endotoxin shock.
Figures








Similar articles
-
Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression.Int J Colorectal Dis. 2003 Jan;18(1):25-32. doi: 10.1007/s00384-002-0415-6. Epub 2002 Jun 14. Int J Colorectal Dis. 2003. PMID: 12458377
-
MD-2 controls bacterial lipopolysaccharide hyporesponsiveness in human intestinal epithelial cells.Life Sci. 2008 Feb 27;82(9-10):519-28. doi: 10.1016/j.lfs.2007.12.007. Epub 2007 Dec 17. Life Sci. 2008. PMID: 18215718
-
Increased expression and internalization of the endotoxin coreceptor CD14 in enterocytes occur as an early event in the development of experimental necrotizing enterocolitis.J Pediatr Surg. 2008 Jun;43(6):1175-81. doi: 10.1016/j.jpedsurg.2008.02.050. J Pediatr Surg. 2008. PMID: 18558203 Free PMC article.
-
Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors.J Immunol. 2000 Jan 15;164(2):966-72. doi: 10.4049/jimmunol.164.2.966. J Immunol. 2000. PMID: 10623846
-
Co-culture Caco-2/Immune Cells.In: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D, Wichers H, editors. The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet]. Cham (CH): Springer; 2015. Chapter 18. In: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D, Wichers H, editors. The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet]. Cham (CH): Springer; 2015. Chapter 18. PMID: 29787066 Free Books & Documents. Review.
Cited by
-
TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling.Cell Mol Life Sci. 2021 Feb;78(4):1233-1261. doi: 10.1007/s00018-020-03656-y. Epub 2020 Oct 15. Cell Mol Life Sci. 2021. PMID: 33057840 Free PMC article. Review.
-
Increased expression of chemokine receptors CCR1 and CCR3 in nasal polyps: molecular basis for recruitment of the granulocyte infiltrate.Folia Microbiol (Praha). 2013 May;58(3):219-24. doi: 10.1007/s12223-012-0194-6. Epub 2012 Oct 11. Folia Microbiol (Praha). 2013. PMID: 23054685
-
Immune cell-mediated protection of the mammary gland and the infant during breastfeeding.Adv Nutr. 2015 May 15;6(3):267-75. doi: 10.3945/an.114.007377. Print 2015 May. Adv Nutr. 2015. PMID: 25979492 Free PMC article. Review.
-
Integrin-mediated first signal for inflammasome activation in intestinal epithelial cells.J Immunol. 2014 Aug 1;193(3):1373-82. doi: 10.4049/jimmunol.1400145. Epub 2014 Jun 25. J Immunol. 2014. PMID: 24965773 Free PMC article.
-
An assessment of potential biomarkers of environment enteropathy and its association with age and microbial infections among children in Bangladesh.PLoS One. 2021 Apr 22;16(4):e0250446. doi: 10.1371/journal.pone.0250446. eCollection 2021. PLoS One. 2021. PMID: 33886672 Free PMC article.
References
-
- Akashi S, Ogata H, Kirikae F, Kirikae T, Kawasaki K, Nishijima M, Shimazu R, Nagai Y, Fukudome K, Kimoto M, Miyake K. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4–MD-2. Biochem Biophys Res Commun. 2000;268:172–177. - PubMed
-
- Bažil V, Baudyš M, Hilgert I, Stefanová I, Low M G, Zbrozek J, Hořejší V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. - PubMed
-
- Bažil V, Strominger J L. Shedding as a mechanism of down-regulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. - PubMed
-
- Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H C, Podolsky D K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. - PubMed
-
- Cebecauer, M., J. Černý, and V. Hořejší. Incorporation of leucocyte GPI-anchored proteins and protein tyrosinc kinases into lipid-rich membrane domains of COS-7 cells. Biochem. Biophys. Res. Commun. 243:706–710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

