Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes
- PMID: 11349069
- PMCID: PMC98462
- DOI: 10.1128/IAI.69.6.3995-4006.2001
Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes
Abstract
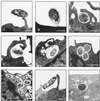
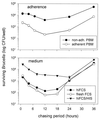
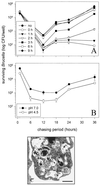
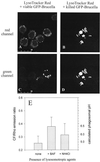
Brucella spp. are facultative intracellular parasites of various mammals, including humans, typically infecting lymphoid as well as reproductive organs. We have investigated how B. suis and B. melitensis enter human monocytes and in which compartment they survive. Peripheral blood monocytes readily internalized nonopsonized brucellae and killed most of them within 12 to 18 h. The presence of Brucella-specific antibodies (but not complement) increased the uptake of bacteria without increasing their intracellular survival, whereas adherence of the monocytes or incubation in Ca(2+)- and Mg(2+)-free medium reduced the uptake. Engulfment of all Brucella organisms (regardless of bacterial viability or virulence) initially resulted in phagosomes with tightly apposed walls (TP). Most TP were fully fusiogenic and matured to spacious phagolysosomes containing degraded bacteria, whereas some TP (more in monocyte-derived macrophages, HeLa cells, and CHO cells than in monocytes) remained tightly apposed to intact bacteria. Immediate treatment of infected host cells with the lysosomotropic base ammonium chloride caused a swelling of all phagosomes and a rise in the intraphagosomal pH, abolishing the intracellular survival of Brucella. These results indicate that (i) human monocytes readily internalize Brucella in a conventional way using various phagocytosis-promoting receptors, (ii) the maturation of some Brucella phagosomes is passively arrested between the steps of acidification and phagosome-lysosome fusion, (iii) brucellae are killed in maturing but not in arrested phagosomes, and (iv) survival of internalized Brucella depends on an acidic intraphagosomal pH and/or close contact with the phagosomal wall.
Figures



Similar articles
-
Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes.Infect Immun. 2005 Jun;73(6):3702-13. doi: 10.1128/IAI.73.6.3702-3713.2005. Infect Immun. 2005. PMID: 15908400 Free PMC article.
-
Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes.J Leukoc Biol. 2003 Dec;74(6):1045-55. doi: 10.1189/jlb.0103015. Epub 2003 Aug 21. J Leukoc Biol. 2003. PMID: 12960272
-
Early events and implication of F-actin and annexin I associated structures in the phagocytic uptake of Brucella suis by the J-774A.1 murine cell line and human monocytes.Microb Pathog. 2000 Jun;28(6):343-52. doi: 10.1006/mpat.2000.0354. Microb Pathog. 2000. PMID: 10839971
-
Interactions between professional phagocytes and Brucella spp.Microbiologia. 1996 Jun;12(2):197-206. Microbiologia. 1996. PMID: 8767704 Review.
-
Brucella stationary-phase gene expression and virulence.Annu Rev Microbiol. 2003;57:57-76. doi: 10.1146/annurev.micro.57.030502.090803. Epub 2003 May 1. Annu Rev Microbiol. 2003. PMID: 12730323 Review.
Cited by
-
Host cell autophagy in immune response to zoonotic infections.Clin Dev Immunol. 2012;2012:910525. doi: 10.1155/2012/910525. Epub 2011 Oct 29. Clin Dev Immunol. 2012. PMID: 22110539 Free PMC article. Review.
-
Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes.Infect Immun. 2005 Jun;73(6):3702-13. doi: 10.1128/IAI.73.6.3702-3713.2005. Infect Immun. 2005. PMID: 15908400 Free PMC article.
-
Evaluation of Th2 and Th17 Immunity-Related Factors as Indicators of Brucellosis.Front Cell Infect Microbiol. 2022 Jan 7;11:786994. doi: 10.3389/fcimb.2021.786994. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35071039 Free PMC article.
-
Circulating Th1, Th2, Th17, Treg, and PD-1 Levels in Patients with Brucellosis.J Immunol Res. 2019 Aug 6;2019:3783209. doi: 10.1155/2019/3783209. eCollection 2019. J Immunol Res. 2019. PMID: 31467933 Free PMC article.
-
Release of periplasmic proteins of Brucella suis upon acidic shock involves the outer membrane protein Omp25.Infect Immun. 2004 Oct;72(10):5693-703. doi: 10.1128/IAI.72.10.5693-5703.2004. Infect Immun. 2004. PMID: 15385468 Free PMC article.
References
-
- Ackermann M, Cheville N, Deyoe B. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet Pathol. 1988;25:28–35. - PubMed
-
- Barclay N, Brown M, Law S, McKnight J, Tomlinson M, van der Merwe P. The leucocyte antigen facts book. 2nd ed. San Diego, Calif: Academic Press; 1997.
-
- Bounous D, Enright F, Gossett K, Berry C. Phagocytosis, killing, and oxidant production by bovine monocyte-derived macrophages upon exposure to Brucella abortus strain 2308. Vet Immunol Immunopathol. 1993;37:243–256. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

