EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri
- PMID: 11349072
- PMCID: PMC98465
- DOI: 10.1128/IAI.69.6.4027-4033.2001
EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri
Abstract
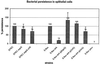
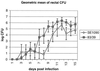
The function of the rorf2 gene located on the locus of enterocyte effacement (LEE) pathogenicity island of enteropathogenic Escherichia coli (EPEC) has not been described. We report that rorf2 encodes a novel protein, named EspG, which is secreted by the type III secretory system and which is translocated into host epithelial cells. EspG is homologous with Shigella flexneri protein VirA, and the cloned espG (rorf2) gene can rescue invasion in a Shigella virA mutant, indicating that these proteins are functionally equivalent in Shigella. An EPEC espG mutant had no apparent defects in in vitro assays of virulence phenotypes, but a rabbit diarrheagenic E. coli strain carrying a mutant espG showed diminished intestinal colonization and yet diarrheal attack rates similar to those of the wild type. A second EspG homolog, Orf3, is encoded on the EspC pathogenicity islet. The cloned orf3 gene could also rescue invasion in a Shigella virA mutant, but an EPEC espG orf3 double mutant was not diminished in any tested in vitro assays for EPEC virulence factors. Our results indicate that EspG plays an accessory but as yet undefined role in EPEC virulence that may involve intestinal colonization.
Figures

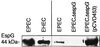

References
-
- Adams L M, Simmons C P, Rezmann L, Strugnell R A, Robins-Browne R M. Identification and characterization of a K88- and CS31A-like operon of a rabbit enteropathogenic Escherichia coli strain which encodes fimbriae involved in the colonization of rabbit intestine. Infect Immun. 1997;65:5222–5230. - PMC - PubMed
-
- Anderson R J, Pasetti M F, Sztein M B, Levine M M, Noriega F R. ΔguaBA attenuated Shigella flexneri 2a strain CVD 1204 as a Shigella vaccine and as a live mucosal delivery system for fragment C of tetanus toxin. Vaccine. 2000;18:2193–2202. - PubMed
-
- Deibel C, Krämer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. - PubMed
-
- Donnenberg S M, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989;160:452–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

