Trichinella spiralis-infected muscle cells: abundant RNA polymerase II in nuclear speckle domains colocalizes with nuclear antigens
- PMID: 11349077
- PMCID: PMC98470
- DOI: 10.1128/IAI.69.6.4065-4071.2001
Trichinella spiralis-infected muscle cells: abundant RNA polymerase II in nuclear speckle domains colocalizes with nuclear antigens
Abstract
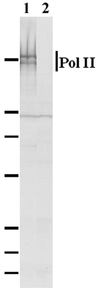
Infection of mammalian skeletal muscle cells by Trichinella spiralis causes host nuclei to become polyploid (ca. 4N) and abnormally enlarged. It has been postulated that this enlargement reflects an infection-induced elevation of host transcription. Anthelmintic treatment of T. spiralis-infected rodents with mebendazole (MBZ) causes a reduction in the size of infected cell nuclei and a significant reduction in the total RNA content of individual infected muscle cells. A monoclonal antibody to the large subunit of RNA polymerase II (Pol II) was used here to assess the effects of infection on Pol II levels in isolated infected cell nuclei. Pol II was localized to speckle domains in isolated infected cell nuclei. Similar domains have been previously localized to sites of RNA synthesis or processing. When compared to the levels in nuclei from other, uninfected host cells, speckle-localized Pol II (SL-Pol II) levels were significantly elevated in infected cell nuclei by a mean of 3.9- to 6.8-fold. Nuclear antigens (NA) recognized by antibodies against T. spiralis localized to infected cell nuclei. By use of confocal microscopy, a subpopulation of NA was found colocalized with most speckle domains defined by Pol II. MBZ treatment of chronically infected mice, which depletes NA from infected cell nuclei, caused a significant depletion of SL-Pol II from infected cell nuclei. Control nuclei had a mean of 70% more SL-Pol II than MBZ-treated nuclei. The mean residual level of Pol II in these polyploid nuclei remained elevated by 120% over the level in 2N control nuclei. These observations may indicate two distinct effects of infection on Pol II levels in host cells.
Figures
Similar articles
-
Host nuclear abnormalities and depletion of nuclear antigens induced in Trichinella spiralis-infected muscle cells by the anthelmintic mebendazole.Mol Biochem Parasitol. 1998 Oct 30;96(1-2):1-13. doi: 10.1016/s0166-6851(98)00082-6. Mol Biochem Parasitol. 1998. PMID: 9851602
-
Nuclear antigens in Trichinella spiralis infected muscle cells: nuclear extraction, compartmentalization and complex formation.Mol Biochem Parasitol. 1998 May 1;92(2):207-18. doi: 10.1016/s0166-6851(97)00199-0. Mol Biochem Parasitol. 1998. PMID: 9657326
-
Failure to detect Trichinella spiralis p43 in isolated host nuclei and in irradiated larvae of infected muscle cells which express the infected cell phenotype.Mol Biochem Parasitol. 1994 Oct;67(2):225-34. doi: 10.1016/0166-6851(94)00131-6. Mol Biochem Parasitol. 1994. PMID: 7870127
-
Effect of Muscle Strength by Trichinella spiralis Infection during Chronic Phase.Int J Med Sci. 2018 May 22;15(8):802-807. doi: 10.7150/ijms.23497. eCollection 2018. Int J Med Sci. 2018. PMID: 30008590 Free PMC article.
-
The role of the host cell nucleus in vaccinia virus morphogenesis.Virus Res. 1987 Sep;8(3):173-91. doi: 10.1016/0168-1702(87)90014-1. Virus Res. 1987. PMID: 3318210 Review.
Cited by
-
Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinases 1 (TIMP-1) are localized in the nucleus of retinal Müller glial cells and modulated by cytokines and oxidative stress.PLoS One. 2021 Jul 16;16(7):e0253915. doi: 10.1371/journal.pone.0253915. eCollection 2021. PLoS One. 2021. PMID: 34270579 Free PMC article.
-
Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair.Parasit Vectors. 2008 Aug 19;1(1):27. doi: 10.1186/1756-3305-1-27. Parasit Vectors. 2008. PMID: 18710582 Free PMC article.
References
-
- Capo V A, Despommier D D, Polvere R I. Trichinella spiralis: vascular endothelial growth factor is up-regulated within the nurse cell during the early phase of its formation. J Parasitol. 1998;84:209–214. - PubMed
-
- Despommier D, Symmans W F, Dell R. Changes in nurse cell nuclei during synchronous infection with Trichinella spiralis. J Parasitol. 1991;77:290–295. - PubMed
-
- Despommier D D. How does Trichinella spiralis make itself at home? Parasitol Today. 1998;14:318–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

