Medium-chain acyl-CoA dehydrogenase (MCAD) mutations identified by MS/MS-based prospective screening of newborns differ from those observed in patients with clinical symptoms: identification and characterization of a new, prevalent mutation that results in mild MCAD deficiency
- PMID: 11349232
- PMCID: PMC1226127
- DOI: 10.1086/320602
Medium-chain acyl-CoA dehydrogenase (MCAD) mutations identified by MS/MS-based prospective screening of newborns differ from those observed in patients with clinical symptoms: identification and characterization of a new, prevalent mutation that results in mild MCAD deficiency
Abstract
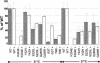
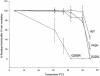
Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency is the most frequently diagnosed mitochondrial beta-oxidation defect, and it is potentially fatal. Eighty percent of patients are homozygous for a common mutation, 985A-->G, and a further 18% have this mutation in only one disease allele. In addition, a large number of rare disease-causing mutations have been identified and characterized. There is no clear genotype-phenotype correlation. High 985A-->G carrier frequencies in populations of European descent and the usual avoidance of recurrent disease episodes by patients diagnosed with MCAD deficiency who comply with a simple dietary treatment suggest that MCAD deficiency is a candidate in prospective screening of newborns. Therefore, several such screening programs employing analysis of acylcarnitines in blood spots by tandem mass spectrometry (MS/MS) are currently used worldwide. No validation of this method by mutation analysis has yet been reported. We investigated for MCAD mutations in newborns from US populations who had been identified by prospective MS/MS-based screening of 930,078 blood spots. An MCAD-deficiency frequency of 1/15,001 was observed. Our mutation analysis shows that the MS/MS-based method is excellent for detection of MCAD deficiency but that the frequency of the 985A-->G mutant allele in newborns with a positive acylcarnitine profile is much lower than that observed in clinically affected patients. Our identification of a new mutation, 199T-->C, which has never been observed in patients with clinically manifested disease but was present in a large proportion of the acylcarnitine-positive samples, may explain this skewed ratio. Overexpression experiments showed that this is a mild folding mutation that exhibits decreased levels of enzyme activity only under stringent conditions. A carrier frequency of 1/500 in the general population makes the 199T-->C mutation one of the three most prevalent mutations in the enzymes of fatty-acid oxidation.
Figures

References
Electronic-Database Information
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MCAD [MIM 201450])
References
-
- Andresen BS, Bross P, Jensen TG, Winter V, Knudsen I, Kølvraa S, Jensen UB, Bolund L, Duran M, Kim JJ, Curtis D, Divry P, Vianey-Saban C, Gregersen N (1993a) A rare disease-associated mutation in the medium-chain acyl-CoA dehydrogenase (MCAD) gene changes a conserved arginine, previously shown to be functionally essential in short-chain acyl-CoA dehydrogenase (SCAD). Am J Hum Genet 53:730–739 - PMC - PubMed
-
- Andresen BS, Bross P, Udvari S, Kirk J, Gray G, Kmoch S, Chamoles N, Knudsen I, Winter V, Wilcken B, Yokota I, Hart K, Packman S, Harpey JP, Saudubray JM, Hale DE, Bolund L, Kølvraa S, Gregersen N (1997) The molecular basis of medium-chain acyl-CoA dehydrogenase (MCAD) deficiency in compound heterozygous patients: Is there correlation between genotype and phenotype? Hum Mol Genet 6:695–707 - PubMed
-
- Andresen BS, Dobrowolski SF, Knudsen I, Banas R, Chace DH, Naylor E, Gregersen N (1999) Molecular genetic validation of the tandem MS method used for newborn screening for MCAD deficiency. J Inher Metab Dis Suppl 22:136
-
- Andresen BS, Dobrowolski SF, O’Reilly L, Engel P, Knudsen I, Banas R, Chace DH, Naylor E, Gregersen N (2000) The mutational spectrum in the MCAD gene of newborns identified by prospective tandem MS screening for “diagnostic” acyl-carnitines in blood spots differs from that observed in clinically affected patients. J Inherit Metab Dis Suppl 23:12
-
- Andresen BS, Kølvraa S, Bross P, Bolund L, Curtis D, Eiberg H, Zhang Z, Kelly DP, Strauss AW, Gregersen N (1993b) A silent A to G mutation in exon 11 of the medium-chain acyl-CoA dehydrogenase (MCAD) gene. Hum Mol Genet 2:488 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

