Chemokine receptor homo- or heterodimerization activates distinct signaling pathways
- PMID: 11350939
- PMCID: PMC125458
- DOI: 10.1093/emboj/20.10.2497
Chemokine receptor homo- or heterodimerization activates distinct signaling pathways
Abstract
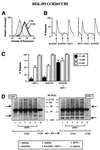
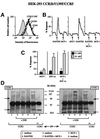
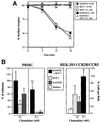
Chemokine receptors of both the CC and CXC families have been demonstrated to undergo a ligand-mediated homodimerization process required for Ca2+ flux and chemotaxis. We show that, in the chemokine response, heterodimerization is also permitted between given receptor pairs, specifically between CCR2 and CCR5. This has functional consequences, as the CCR2 and CCR5 ligands monocyte chemotactic protein-1 (MCP-1) and RANTES (regulated upon activation, normal T cell-expressed and secreted) cooperate to trigger calcium responses at concentrations 10- to 100-fold lower than the threshold for either chemokine alone. Heterodimerization results in recruitment of each receptor-associated signaling complex, but also recruits dissimilar signaling path ways such as G(q/11) association, and delays activation of phosphatidyl inositol 3-kinase. The consequences are a pertussis toxin-resistant Ca2+ flux and trig gering of cell adhesion rather than chemotaxis. These results show the effect of heterodimer formation on increasing the sensitivity and dynamic range of the chemokine response, and may aid in understanding the dynamics of leukocytes at limiting chemokine concentrations in vivo.
Figures





References
-
- Al-Aoukaty A., Schall,T.J. and Maghazachi,A.A. (1996) Differential coupling of CC chemokine receptors to multiple heterotrimeric G proteins in human interleukin 2-activated natural killer cells. Blood, 87, 4255–4260. - PubMed
-
- Alon U., Surette,M.G., Barkai,N. and Leibler,S. (1999) Robustness in bacterial chemotaxis. Nature, 397, 168–171. - PubMed
-
- Arai H. and Charo,I.F. (1996) Differential regulation of G-protein mediated signaling by chemokine receptors. J. Biol. Chem., 271, 21814–21819. - PubMed
-
- Baggiolini M. (1998) Chemokines and leukocyte traffic. Nature, 392, 565–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

