Structure and mechanism of the RNA triphosphatase component of mammalian mRNA capping enzyme
- PMID: 11350947
- PMCID: PMC125469
- DOI: 10.1093/emboj/20.10.2575
Structure and mechanism of the RNA triphosphatase component of mammalian mRNA capping enzyme
Abstract
The 5' capping of mammalian pre-mRNAs is initiated by RNA triphosphatase, a member of the cysteine phosphatase superfamily. Here we report the 1.65 A crystal structure of mouse RNA triphosphatase, which reveals a deep, positively charged active site pocket that can fit a 5' triphosphate end. Structural, biochemical and mutational results show that despite sharing an HCxxxxxR(S/T) motif, a phosphoenzyme intermediate and a core alpha/beta-fold with other cysteine phosphatases, the mechanism of phosphoanhydride cleavage by mammalian capping enzyme differs from that used by protein phosphatases to hydrolyze phosphomonoesters. The most significant difference is the absence of a carboxylate general acid catalyst in RNA triphosphatase. Residues conserved uniquely among the RNA phosphatase subfamily are important for function in cap formation and are likely to play a role in substrate recognition.
Figures

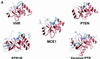
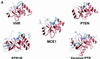






References
-
- Barford D., Flint,A.J. and Tonks,N.K. (1994) Crystal structure of human protein tyrosine phosphatase 1B. Science, 263, 1397–1404. - PubMed
-
- Becker K., Savvides,S.N., Keese,M., Schirmer,R.H. and Karplus,P.A. (1998) Enzyme inactivation through sulfhydryl oxidation by physiologic NO-carriers. Nature Struct. Biol., 5, 267–271. - PubMed
-
- Brünger A.T. et al. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
-
- CCP4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. - PubMed
-
- Chen W., Wilborn,M. and Rudolph,J. (2000) Dual-specific Cdc25B phosphatase: in search of the catalytic acid. Biochemistry, 39, 10781–10789. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

