Neural prostheses
- PMID: 11351018
- PMCID: PMC2278603
- DOI: 10.1111/j.1469-7793.2001.0099b.x
Neural prostheses
Abstract
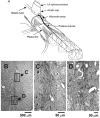
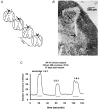
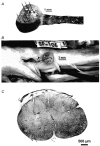
Assuming that neural regeneration after spinal cord injury (SCI) will eventually become a clinical reality, functional recovery will probably remain incomplete. Assistive devices will therefore continue to play an important role in rehabilitation. Neural prostheses (NPs) are assistive devices that restore functions lost as a result of neural damage. NPs electrically stimulate nerves and are either external or implanted devices. Surface stimulators for muscle exercise are now commonplace in rehabilitation clinics and many homes. Regarding implantable NPs, since 1963 over 40 000 have been implanted to restore hearing, bladder control and respiration. Epidural spinal cord stimulators and deep brain stimulators are routinely implanted to control pain, spasticity, tremor and rigidity. Implantable NPs have also been developed to restore limb movements using electrodes tunnelled under the skin to muscles and nerves. Spinal cord microstimulation (SC[mu]stim) is under study as an alternative way of restoring movement and bladder control. Improvement in bladder and bowel function is a high priority for many SCI people. Sacral root stimulation to elicit bladder contraction is the current NP approach, but this usually requires dorsal rhizotomies to reduce reflex contractions of the external urethral sphincter. It is possible that the spinal centres coordinating the bladder-sphincter synergy could be activated with SC[mu]stim. Given the large and growing number of NPs in use or development, it is surprising how little is known about their long-term interactions with the nervous system. Physiological research will play an important role in elucidating the mechanisms underlying these interactions.
Figures
References
-
- Benabid A, Koudsie A, Benazzouz A, Fraix V, Ashraf A, Le bas JF, Chabardes S, Pollak P. Subthalamic stimulation for Parkinson's disease. Archives of Medical Research. 2000;31:282–289. - PubMed
-
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. - PubMed
-
- Blok BFM, Holstege G. The central nervous system control of micturition in cats and humans. Behavioural Brain Research. 1998;92:119–125. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

