Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres
- PMID: 11351027
- PMCID: PMC2278591
- DOI: 10.1111/j.1469-7793.2001.0185b.x
Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres
Abstract
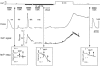
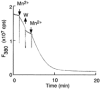
To examine whether a capacitative Ca2+ entry pathway is present in skeletal muscle, thin muscle fibre bundles were isolated from extensor digitorum longus (EDL) muscle of adult mice, and isometric tension and fura-2 signals were simultaneously measured. The sarcoplasmic reticulum (SR) in the muscle fibres was successfully depleted of Ca2+ by repetitive treatments with high-K+ solutions, initially in the absence and then in the presence of a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor. Depletion of the SR of Ca2+ enabled us for the first time to show convincingly that the vast majority of the voltage-sensitive Ca2+ store overlaps the caffeine-sensitive Ca2+ store in intact fibres from mouse EDL muscle. This conclusion was based on the observation that both high-K+ solution and caffeine failed to cause a contracture in the depleted muscle fibres. The existence of a Ca2+ influx pathway active enough to refill the depleted SR within several minutes was shown in skeletal muscle fibres. Ca2+ entry was sensitive to Ni2+, but resistant to nifedipine and was suppressed by plasma membrane depolarisation. Evidence for store-operated Ca2+ entry was provided by measurements of Mn2+ entry. Significant acceleration of Mn2+ entry was observed only when the SR was severely depleted of Ca2+. The Mn2+ influx, which was blocked by Ni2+ but not by nifedipine, was inwardly rectifying, as is the case with the Ca2+ entry. These results indicate that the store-operated Ca2+ entry is similar to the Ca2+ release-activated Ca2+ channel (CRAC) current described in other preparations.
Figures






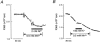
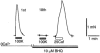
Similar articles
-
Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat.J Physiol. 2009 Jan 15;587(2):443-60. doi: 10.1113/jphysiol.2008.163162. Epub 2008 Nov 24. J Physiol. 2009. PMID: 19029185 Free PMC article.
-
Cyclopiazonic acid and thapsigargin reduce Ca2+ influx in frog skeletal muscle fibres as a result of Ca2+ store depletion.Acta Physiol Scand. 2001 Dec;173(4):391-9. doi: 10.1046/j.1365-201X.2001.00918.x. Acta Physiol Scand. 2001. PMID: 11903131
-
Sarcoplasmic reticulum Ca2+ release and depletion fail to affect sarcolemmal ion channel activity in mouse skeletal muscle.J Physiol. 2006 Aug 15;575(Pt 1):69-81. doi: 10.1113/jphysiol.2006.112367. Epub 2006 Jun 15. J Physiol. 2006. PMID: 16777939 Free PMC article.
-
Capacitative Ca2+ entry and the regulation of smooth muscle tone.Trends Pharmacol Sci. 1998 Jul;19(7):266-9. doi: 10.1016/s0165-6147(98)01222-x. Trends Pharmacol Sci. 1998. PMID: 9703759 Review.
-
Calcium uptake and release modulated by counter-ion conductances in the sarcoplasmic reticulum of skeletal muscle.Acta Physiol Scand. 1996 Mar;156(3):387-96. doi: 10.1046/j.1365-201X.1996.212000.x. Acta Physiol Scand. 1996. PMID: 8729699 Review.
Cited by
-
Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy.Nat Rev Cardiol. 2012 Dec;9(12):717-33. doi: 10.1038/nrcardio.2012.145. Epub 2012 Oct 23. Nat Rev Cardiol. 2012. PMID: 23090087 Free PMC article. Review.
-
Excitation-contraction coupling in mammalian skeletal muscle: Blending old and last-decade research.Front Physiol. 2022 Sep 2;13:989796. doi: 10.3389/fphys.2022.989796. eCollection 2022. Front Physiol. 2022. PMID: 36117698 Free PMC article. Review.
-
Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers.J Cell Biol. 2002 Sep 16;158(6):1089-96. doi: 10.1083/jcb.200203091. Epub 2002 Sep 16. J Cell Biol. 2002. PMID: 12235126 Free PMC article.
-
Physiological and Pathological Relevance of Selective and Nonselective Ca2+ Channels in Skeletal and Cardiac Muscle.Adv Exp Med Biol. 2021;1349:225-247. doi: 10.1007/978-981-16-4254-8_11. Adv Exp Med Biol. 2021. PMID: 35138617 Free PMC article.
-
Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue.Nat Commun. 2013;4:2805. doi: 10.1038/ncomms3805. Nat Commun. 2013. PMID: 24241282 Free PMC article.
References
-
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(β-aminoethyl ether)-N,N′-tetracetic acid. Biochimica et Biophysica Acta. 1972;267:605–608. - PubMed
-
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–67. - PubMed
-
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proceedings of the National Academy of Sciences of the USA. 1996;93:15195–15202. - PMC - PubMed
-
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proceedings of the National Academy of Sciences of the USA. 1999;96:14955–14960. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

