The cortical deficit in humans with strabismic amblyopia
- PMID: 11351035
- PMCID: PMC2278601
- DOI: 10.1111/j.1469-7793.2001.0281b.x
The cortical deficit in humans with strabismic amblyopia
Abstract
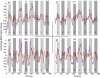
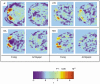
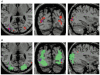
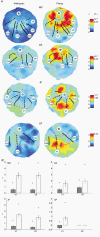
To further our understanding of the cortical deficit in strabismic amblyopia, we measured, compared and mapped functional magnetic resonance imaging (fMRI) activation between the fixing and fellow amblyopic eyes of ten strabismic amblyopes. Of specific concern was whether the function of any visual area was spared in strabismic amblyopia, as recently suggested by both positron emission tomography (PET) and fMRI studies, and whether there was a close relationship between the fMRI response and known psychophysical deficits. To answer these questions we measured the psychophysical deficit in each subject and used stimuli whose relationship to the psychophysical deficit was known. We observed that stimuli that were well within the amblyopic passband did produce reduced fMRI activation, even in visual area V1. This suggests that V1 is anomalous in amblyopia. A similar level of reduction was observed in V2. In two subjects, we found that stimuli outside the amblyopic passband produced activation in visual area V3A. We did not find a close relationship between the fMRI response reduction in amblyopia and either of the known psychophysical deficits even though the fMRI response reduction in amblyopia did covary with stimulus spatial frequency.
Figures



References
-
- Anderson SJ, Holliday IE, Harding GFA. Assessment of cortical dysfunction in human strabismic amblyopia using magnetoencephalography (MEG) Vision Research. 1999;39:1723–1738. - PubMed
-
- Arden GB, Barnard WB. Effect of occlusion on the visually evoked potential in amblyopia. Transactions of the Ophthalmological Society, UK. 1979;99:419–426. - PubMed
-
- Baker FH, Grigg P, von Noorden GK. Effects of visual deprivation and strabismus on the response of neurons in the visual cortex of the monkey, including studies of striate and pre-striate cortex in normal animals. Brain Research. 1974;66:185–208.
-
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. - PubMed
-
- Bedell HD, Flom MC. Monocular spatial distortion in strabismic amblyopia. Investigative Ophthalmology and Visual Science. 1981;20:263–268. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

