An early tobacco mosaic virus-induced oxidative burst in tobacco indicates extracellular perception of the virus coat protein
- PMID: 11351074
- PMCID: PMC102285
- DOI: 10.1104/pp.126.1.97
An early tobacco mosaic virus-induced oxidative burst in tobacco indicates extracellular perception of the virus coat protein
Abstract
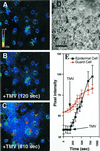
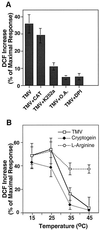
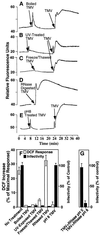
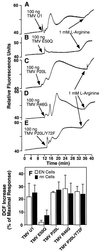
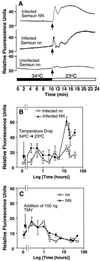
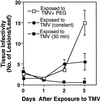
Induction of reactive oxygen species (ROS) was observed within seconds of the addition of exogenous tobacco mosaic virus (TMV) to the outside of tobacco (Nicotiana tabacum cv Samsun NN, EN, or nn) epidermal cells. Cell death was correlated with ROS production. Infectivity of the TMV virus was not a prerequisite for this elicitation and isolated coat protein (CP) subunits could also elicit the fast oxidative burst. The rapid induction of ROS was prevented by both inhibitors of plant signal transduction and inhibitors of NAD(P)H oxidases, suggesting activation of a multi-step signal transduction pathway. Induction of intracellular ROS by TMV was detected in TMV-resistant and -susceptible tobacco cultivars isogenic for the N allele. The burst was also detected with strains of virus that either elicit (ToMV) or fail to elicit (TMV U1) N' gene-mediated responses. Hence, early ROS generation is independent or upstream of known genetic systems in tobacco that can mediate hypersensitive responses. Analysis of other viruses and TMV CP mutants showed marked differences in their ability to induce ROS showing specificity of the response. Thus, initial TMV-plant cell interactions that lead to early ROS induction occur outside the plasma membrane in an event requiring specific CP epitopes.
Figures


References
-
- Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar SP. Signaling in plant-microbe interactions. Science. 1997;276:726–733. - PubMed
-
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Annu Rev Phytopathol. 1995;33:299–321. - PubMed
-
- Bolwell GP, Buti VS, Davies DR, Zimmerlin A. The origin of the oxidative burst in plants. Free Radic Res. 1995;23:517–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

