The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for plasmodium sporozoite attachment to target cells
- PMID: 11352923
- PMCID: PMC3941197
- DOI: 10.1074/jbc.M104038200
The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for plasmodium sporozoite attachment to target cells
Abstract
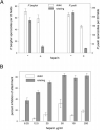
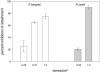
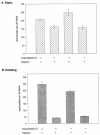
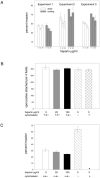
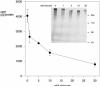
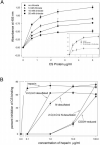
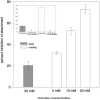
The major surface protein of malaria sporozoites, the circumsporozoite protein, binds to heparan sulfate proteoglycans on the surface of hepatocytes. It has been proposed that this binding event is responsible for the rapid and specific localization of sporozoites to the liver after their injection into the skin by an infected anopheline mosquito. Previous in vitro studies performed under static conditions have failed to demonstrate a significant role for heparan sulfate proteoglycans during sporozoite invasion of cells. We performed sporozoite attachment and invasion assays under more dynamic conditions and found a dramatic decrease in sporozoite attachment to cells in the presence of heparin. In contrast to its effect on attachment, heparin does not appear to have an effect on sporozoite invasion of cells. When substituted heparins were used as competitive inhibitors of sporozoite attachment, we found that sulfation of the glycosaminoglycan chains at both the N- and O-positions was important for sporozoite adhesion to cells. We conclude that the binding of the circumsporozoite protein to hepatic heparan sulfate proteoglycans is likely to function during sporozoite attachment in the liver and that this adhesion event depends on the sulfated glycosaminoglycan chains of the proteoglycans.
Figures

References
-
- Renia L, Miltgen F, Charoenvit Y, Ponnudurai T, Verhave JP, Collins WE, Mazier D. J. Immunol. Methods. 1988;112:201–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

