The drosophila protein asp is involved in microtubule organization during spindle formation and cytokinesis
- PMID: 11352927
- PMCID: PMC2192390
- DOI: 10.1083/jcb.153.4.637
The drosophila protein asp is involved in microtubule organization during spindle formation and cytokinesis
Abstract
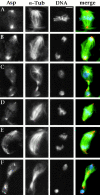
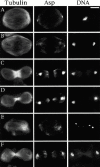
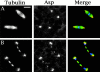
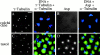
Abnormal spindle (Asp) is a 220-kD microtubule-associated protein from Drosophila that has been suggested to be involved in microtubule nucleation from the centrosome. Here, we show that Asp is enriched at the poles of meiotic and mitotic spindles and localizes to the minus ends of central spindle microtubules. Localization to these structures is independent of a functional centrosome. Moreover, colchicine treatment disrupts Asp localization to the centrosome, indicating that Asp is not an integral centrosomal protein. In both meiotic and mitotic divisions of asp mutants, microtubule nucleation occurs from the centrosome, and gamma-tubulin localizes correctly. However, spindle pole focusing and organization are severely affected. By examining cells that carry mutations both in asp and in asterless, a gene required for centrosome function, we have determined the role of Asp in the absence of centrosomes. Phenotypic analysis of these double mutants shows that Asp is required for the aggregation of microtubules into focused spindle poles, reinforcing the conclusion that its function at the spindle poles is independent of any putative role in microtubule nucleation. Our data also suggest that Asp has a role in the formation of the central spindle. The inability of asp mutants to correctly organize the central spindle leads to disruption of the contractile ring machinery and failure in cytokinesis.
Figures

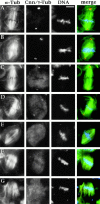

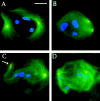
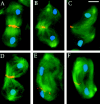
References
-
- Avides M.C., Glover D.M. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 1999;283:1733–1735. - PubMed
-
- Bonaccorsi S., Giansanti M.G., Cenci G., Gatti M. Cytological analysis of spermatocyte growth and male meiosis in Drosophila melanogaster. Sullivan W., Ashburner M., Hawley R.S. Drosophila Protocols 2000. 87 109 Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: a
-
- Bonaccorsi S., Giansanti M.G., Gatti M. Spindle assembly in Drosophila neuroblasts and ganglion mother cells Nat. Cell Biol. 2 2000. 54 56b - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

