Alterations at the intercalated disk associated with the absence of muscle LIM protein
- PMID: 11352937
- PMCID: PMC2192386
- DOI: 10.1083/jcb.153.4.763
Alterations at the intercalated disk associated with the absence of muscle LIM protein
Abstract
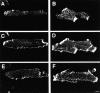
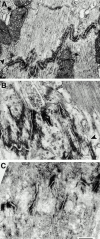
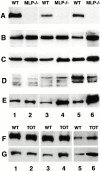
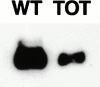
In this study, we investigated cardiomyocyte cytoarchitecture in a mouse model for dilated cardiomyopathy (DCM), the muscle LIM protein (MLP) knockout mouse and substantiated several observations in a second DCM model, the tropomodulin-overexpressing transgenic (TOT) mouse. Freshly isolated cardiomyocytes from both strains are characterized by a more irregular shape compared with wild-type cells. Alterations are observed at the intercalated disks, the specialized areas of mechanical coupling between cardiomyocytes, whereas the subcellular organization of contractile proteins in the sarcomeres of MLP knockout mice appears unchanged. Distinct parts of the intercalated disks are affected differently. Components from the adherens junctions are upregulated, desmosomal proteins are unchanged, and gap junction proteins are downregulated. In addition, the expression of N-RAP, a LIM domain- containing protein located at the intercalated disks, is upregulated in MLP knockout as well as in TOT mice. Detailed analysis of intercalated disk composition during postnatal development reveals that an upregulation of N-RAP expression might serve as an early marker for the development of DCM. Altered expression levels of cytoskeletal proteins (either the lack of MLP or an increased expression of tropomodulin) apparently lead to impaired function of the myofibrillar apparatus and to physiological stress that ultimately results in DCM and is accompanied by an altered appearance and composition of the intercalated disks.
Figures




References
-
- Angst B.D., Khan L.U., Severs N.J., Whitely K., Rothery S., Thompson R.P., Magee A.I., Gourdie R.G. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ. Res. 1997;80:88–94. - PubMed
-
- Arber S., Caroni P. Specificity of single LIM motifs in targeting and LIM/LIM interactions in situ. Genes Dev. 1996;10:289–300. - PubMed
-
- Arber S., Halder G., Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79:221–231. - PubMed
-
- Arber S., Hunter J.J., Ross J.J., Hongo M., Sansig G., Borg J., Perriard J.-C., Chien K.R., Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. - PubMed
-
- Bähler M., Moser H., Eppenberger H.M., Wallimann T. Heart c-protein is transiently expressed during skeletal muscle development in the embryo, but persists in cultured myogenic cells. Dev. Biol. 1985;112:345–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

