Role of phosphatidylinositol 3' kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in dictyostelium
- PMID: 11352940
- PMCID: PMC2192389
- DOI: 10.1083/jcb.153.4.795
Role of phosphatidylinositol 3' kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in dictyostelium
Abstract
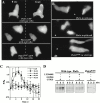
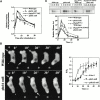
We show that cells lacking two Dictyostelium class I phosphatidylinositol (PI) 3' kinases (PI3K and pi3k1/2-null cells) or wild-type cells treated with the PI3K inhibitor LY294002 are unable to properly polarize, are very defective in the temporal, spatial, and quantitative regulation of chemoattractant-mediated filamentous (F)-actin polymerization, and chemotax very slowly. PI3K is thought to produce membrane lipid-binding sites for localization of PH domain-containing proteins. We demonstrate that in response to chemoattractants three PH domain-containing proteins do not localize to the leading edge in pi3k1/2-null cells, and the translocation is blocked in wild-type cells by LY294002. Cells lacking one of these proteins, phdA-null cells, exhibit defects in the level and kinetics of actin polymerization at the leading edge and have chemotaxis phenotypes that are distinct from those described previously for protein kinase B (PKB) (pkbA)-null cells. Phenotypes of PhdA-dominant interfering mutations suggest that PhdA is an adaptor protein that regulates F-actin localization in response to chemoattractants and links PI3K to the control of F-actin polymerization at the leading edge during pseudopod formation. We suggest that PKB and PhdA lie downstream from PI3K and control different downstream effector pathways that are essential for proper chemotaxis.
Figures










References
-
- Andjelkovic M., Alessi D.R., Meier R., Fernandez A., Lamb N.J., Frech M., Cron P., Cohen P., Lucocq J.M., Hemmings B.A. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997;272:31515–31524. - PubMed
-
- Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
-
- Buczynski G., Grove B., Nomura A., Kleve M., Bush J., Firtel R.A., Cardelli J. Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to postlysosome transport, and actin cytoskeleton organization. J. Cell Biol. 1997;136:1271–1286. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

