Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts
- PMID: 11352946
- PMCID: PMC2192381
- DOI: 10.1083/jcb.153.4.881
Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts
Abstract
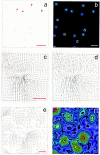
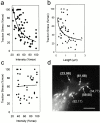
Fibroblast migration involves complex mechanical interactions with the underlying substrate. Although tight substrate contact at focal adhesions has been studied for decades, the role of focal adhesions in force transduction remains unclear. To address this question, we have mapped traction stress generated by fibroblasts expressing green fluorescent protein (GFP)-zyxin. Surprisingly, the overall distribution of focal adhesions only partially resembles the distribution of traction stress. In addition, detailed analysis reveals that the faint, small adhesions near the leading edge transmit strong propulsive tractions, whereas large, bright, mature focal adhesions exert weaker forces. This inverse relationship is unique to the leading edge of motile cells, and is not observed in the trailing edge or in stationary cells. Furthermore, time-lapse analysis indicates that traction forces decrease soon after the appearance of focal adhesions, whereas the size and zyxin concentration increase. As focal adhesions mature, changes in structure, protein content, or phosphorylation may cause the focal adhesion to change its function from the transmission of strong propulsive forces, to a passive anchorage device for maintaining a spread cell morphology.
Figures





References
-
- Abercrombie M., Dunn G.A. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp. Cell Res. 1975;92:57–62. - PubMed
-
- Beckerle M.C. Zyxin, zinc fingers at sites of cell adhesions. Bioessays. 1997;19:949–957. - PubMed
-
- Bershadsky A., Chavsovsky A., Becker E., Lyubimova A., Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 1996;6:1279–1289. - PubMed
-
- Bray, D. 2001. Cell Movement: From Molecules to Motility. M. Day, editor. 2nd ed. Garland Publishing, New York. 400 pp.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

