Treatment of tuberculosis using a combination of sustained-release rifampin-loaded microspheres and oral dosing with isoniazid
- PMID: 11353605
- PMCID: PMC90525
- DOI: 10.1128/AAC.45.6.1637-1644.2001
Treatment of tuberculosis using a combination of sustained-release rifampin-loaded microspheres and oral dosing with isoniazid
Abstract
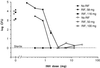
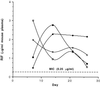
Previously, we reported on the use of rifampin-loaded microspheres to effectively treat Mycobacterium tuberculosis-infected macrophages and mice. Using similar biocompatible polymeric excipients of lactide and glycolide copolymers, we have increased the rifampin loading of small microsphere formulations (1 to 10 microm) by fourfold. Improved formulations were evaluated individually and in combination with oral regimens of isoniazid for the treatment of Mycobacterium tuberculosis H37Rv-infected mice. Groups (10 mice per group) consisted of mice that received (i) oral dosages of isoniazid (25 to 0.19 mg/kg of body weight/day), (ii) two intraperitoneal injections of rifampin-loaded microspheres on days 0 and 7, (iii) a combination of small rifampin-loaded microspheres on days 0 and 7 and isoniazid orally for 25 days (12.5 to 0.39 mg/kg/day), (iv) placebo injections, and (v) no treatment. Treatment with rifampin-loaded microspheres alone resulted in significant reductions in the numbers of CFU in the lungs and spleens by day 26. A bioassay revealed that plasma rifampin levels from the microspheres exceeded the MICs by more than twofold throughout the 26-day experimental period. Susceptibility testing demonstrated continued sensitivity to rifampin during the treatment period. Whereas isoniazid alone significantly reduced the numbers of CFU for dosages ranging from 12.5 to 1.56 mg/kg, combination therapy with rifampin-loaded microspheres increased the effective range to 0.39 mg/kg. In many cases, complete elimination of CFU was obtained with the combination therapy, something not achieved with most of the single therapies. These results demonstrate the ability to use small microsphere formulations alone to achieve significant results in a murine tuberculosis model and also the ability to use them safely in combination with another antimycobacterial agent.
Figures

References
-
- Abazinge M, Jackson T, Yang Q, Owusu-Ababio G. In vitro and in vivo characterization of biodegradable enoxacin microspheres. Eur J Pharm Biopharm. 2000;49:191–194. - PubMed
-
- Arachi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. - PubMed
-
- Bahk J Y, Hyun J S, Lee J Y, Kim J, Cho Y H, Lee J H, Park J S, Kim M O. Concentration of ofloxacin in canine prostate tissue and prostate fluid after intraprostatic injection of biodegradable sustained-releasing microspheres containing ofloxacin. J Urol. 2000;163:1560–1564. - PubMed
-
- Centers for Disease Control and Prevention. CDC revises HIV infection estimates. HIV/AIDS Prevent. 1996;st:2.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

