Teicoplanin stress-selected mutations increasing sigma(B) activity in Staphylococcus aureus
- PMID: 11353616
- PMCID: PMC90536
- DOI: 10.1128/AAC.45.6.1714-1720.2001
Teicoplanin stress-selected mutations increasing sigma(B) activity in Staphylococcus aureus
Abstract
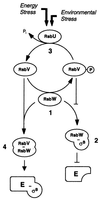
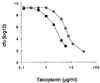
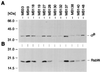
A natural rsbU mutant of Staphylococcus aureus, unable to activate the alternative transcription factor sigma(B) via the RsbU pathway and therefore forming unpigmented colonies, produced first-step teicoplanin-resistant mutants upon selection for growth in the presence of teicoplanin, of which the majority were of an intense orange color. By using an asp23 promoter-luciferase fusion as an indicator, the pigmented mutants were shown to express increased sigma(B) activity. Increased sigma(B) activity was associated with point mutations in rsbW, releasing sigma(B) from sequestration by the anti-sigma factor RsbW, or to promoter mutations increasing the sigma(B)/RsbW ratio. Genetic manipulations involving the sigB operon suggested that the mutations within the operon were associated with the increase in teicoplanin resistance. The upregulation of sigma(B) suggests that a sigma(B)-controlled gene(s) is directly or indirectly involved in the development of teicoplanin resistance in S. aureus. Carotenoids do not contribute to teicoplanin resistance, since inactivation of the dehydrosqualene synthase gene crtM abolished pigment formation without affecting teicoplanin resistance. The relevant sigma(B)-controlled target genes involved in teicoplanin resistance remain to be identified.
Figures


References
-
- Bischoff M, Roos M, Putnik J, Wada A, Glanzmann P, Giachino P, Vaudaux P, Berger-Bächi B. Involvement of multiple genetic loci in Staphylococcus aureusteicoplanin resistance. FEMS Microbiol Lett. 2001;194:77–82. - PubMed
-
- Brückner R. Gene replacement in Staphylococcus camosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

