Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus
- PMID: 11353619
- PMCID: PMC90539
- DOI: 10.1128/AAC.45.6.1737-1742.2001
Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus
Abstract
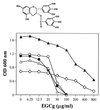
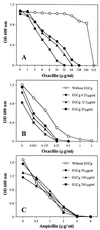
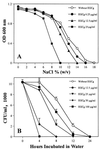
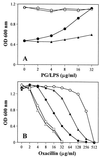
Compared to MICs (more than 800 microg/ml) of (-)-epigallocatechin gallate (EGCg) against Escherchia coli, MICs of EGCg against methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MSSA and MRSA) were 100 microg/ml or less. Furthermore, less than 25 microg EGCg per ml obviously reversed the high level resistance of MRSA to all types of tested beta-lactams, including benzylpenicillin, oxacillin, methicillin, ampicillin, and cephalexin. EGCg also induced a supersusceptibility to beta-lactams in MSSA which does not express mecA, encoding penicillin-binding protein 2' (PBP2'). The fractional inhibitory concentration (FIC) indices of the tested beta-lactams against 25 isolates of MRSA were from 0.126 to 0.625 in combination with 6.25, 12.5 or 25 microg of EGCg per ml. However, no synergism was observed between EGCg and ampicillin against E. coli. EGCg largely reduced the tolerance of MRSA and MSSA to high ionic strength and low osmotic pressure in their external atmosphere, indicating damage of the cell wall. Unlike dextran and lipopolysaccharide, peptidoglycan from S. aureus blocked both the antibacterial activity of EGCg and the synergism between EGCg and oxacillin, suggesting a direct binding of EGCg with peptidoglycan on the cell wall. EGCg showed a synergistic effect with DL-cycloserine (an inhibitor of cell wall synthesis unrelated to PBP2') but additive or indifferent effect with inhibitors of protein and nuclear acid synthesis. EGCg did not suppress either PBP2' mRNA expression or PBP2' production, as confirmed by reverse transcription-PCR and a semiquantitative PBP2' latex agglutination assay, indicating an irrelevance between the synergy and PBP2' production. In summary, both EGCg and beta-lactams directly or indirectly attack the same site, peptidoglycan on the cell wall. EGCg synergizes the activity of beta-lactams against MRSA owing to interference with the integrity of the cell wall through direct binding to peptidoglycan.
Figures


References
-
- Chambers H F. Penicillin-binding protein-mediated resistance in pneumococci and staphylococci. J Infect Dis. 1999;179(Suppl. 2):S353–S359. - PubMed
-
- Chen L, Lee M J, Li H, Yang C S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–1050. - PubMed
-
- de Lencastre H, de Jonge B L, Matthews P R, Tomasz A. Molecular aspects of methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1994;33:7–24. - PubMed
-
- Ghuysen J M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

