Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis
- PMID: 11353624
- PMCID: PMC90544
- DOI: 10.1128/AAC.45.6.1771-1779.2001
Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis
Abstract
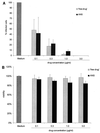
Immunocompromised patients are at risk of developing toxoplasma encephalitis (TE). Standard therapy regimens (including sulfadiazine plus pyrimethamine) are hampered by severe side effects. While atovaquone has potent in vitro activity against Toxoplasma gondii, it is poorly absorbed after oral administration and shows poor therapeutic efficacy against TE. To overcome the low absorption of atovaquone, we prepared atovaquone nanosuspensions (ANSs) for intravenous (i.v.) administration. At concentrations higher than 1.0 microg/ml, ANS did not exert cytotoxicity and was as effective as free atovaquone (i.e., atovaquone suspended in medium) against T. gondii in freshly isolated peritoneal macrophages. In a new murine model of TE that closely mimics reactivated toxoplasmosis in immunocompromised hosts, using mice with a targeted mutation in the gene encoding the interferon consensus sequence binding protein, i.v.-administered ANS doses of 10.0 mg/kg of body weight protected the animals against development of TE and death. Atovaquone was detectable in the sera, brains, livers, and lungs of mice by high-performance liquid chromatography. Development of TE and mortality in mice treated with 1.0- or 0.1-mg/kg i.v. doses of ANS did not differ from that in mice treated orally with 100 mg of atovaquone/kg. In conclusion, i.v. ANSs may prove to be an effective treatment alternative for patients with TE.
Figures




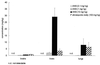
Similar articles
-
Atovaquone maintenance therapy prevents reactivation of toxoplasmic encephalitis in a murine model of reactivated toxoplasmosis.Antimicrob Agents Chemother. 2004 Dec;48(12):4848-54. doi: 10.1128/AAC.48.12.4848-4854.2004. Antimicrob Agents Chemother. 2004. PMID: 15561866 Free PMC article.
-
SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood-brain barriers.J Drug Target. 2011 Feb;19(2):114-24. doi: 10.3109/10611861003733995. Epub 2010 Apr 1. J Drug Target. 2011. PMID: 20367080
-
The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine.J Infect Dis. 1993 Feb;167(2):494-7. doi: 10.1093/infdis/167.2.494. J Infect Dis. 1993. PMID: 8421189
-
Atovaquone: a review.Ann Pharmacother. 1993 Dec;27(12):1488-94. doi: 10.1177/106002809302701215. Ann Pharmacother. 1993. PMID: 8305784 Review.
-
[New pathogens and mode of action of azithromycin: Toxoplasma gondii].Pathol Biol (Paris). 1995 Jun;43(6):561-4. Pathol Biol (Paris). 1995. PMID: 8539083 Review. French.
Cited by
-
Formulation and evaluation of atovaquone-loaded macrophage-derived exosomes against Toxoplasma gondii: in vitro and in vivo assessment.Microbiol Spectr. 2024 Jan 11;12(1):e0308023. doi: 10.1128/spectrum.03080-23. Epub 2023 Nov 28. Microbiol Spectr. 2024. PMID: 38014940 Free PMC article.
-
Prenylquinones in Human Parasitic Protozoa: Biosynthesis, Physiological Functions, and Potential as Chemotherapeutic Targets.Molecules. 2019 Oct 16;24(20):3721. doi: 10.3390/molecules24203721. Molecules. 2019. PMID: 31623105 Free PMC article. Review.
-
Nano-Encapsulated Melatonin: A Promising Mucosal Adjuvant in Intranasal Immunization against Chronic Experimental T. gondii Infection.Trop Med Infect Dis. 2022 Nov 27;7(12):401. doi: 10.3390/tropicalmed7120401. Trop Med Infect Dis. 2022. PMID: 36548656 Free PMC article.
-
Atovaquone maintenance therapy prevents reactivation of toxoplasmic encephalitis in a murine model of reactivated toxoplasmosis.Antimicrob Agents Chemother. 2004 Dec;48(12):4848-54. doi: 10.1128/AAC.48.12.4848-4854.2004. Antimicrob Agents Chemother. 2004. PMID: 15561866 Free PMC article.
-
Nanoparticles containing insoluble drug for cancer therapy.Biotechnol Adv. 2014 Jul-Aug;32(4):778-88. doi: 10.1016/j.biotechadv.2013.10.002. Epub 2013 Oct 8. Biotechnol Adv. 2014. PMID: 24113214 Free PMC article. Review.
References
-
- Alyautdin R, Gothier D, Petrov V, Kharkevich D, Kreuter J. Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butylcyanoacrylate) nanoparticles. Eur J Pharm Biopharm. 1995;41:44–48.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

