Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor
- PMID: 11353823
- PMCID: PMC33412
- DOI: 10.1073/pnas.101126198
Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor
Abstract
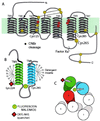
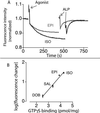
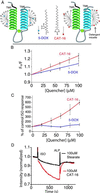
The majority of extracellular physiologic signaling molecules act by stimulating GTP-binding protein (G-protein)-coupled receptors (GPCRs). To monitor directly the formation of the active state of a prototypical GPCR, we devised a method to site specifically attach fluorescein to an endogenous cysteine (Cys-265) at the cytoplasmic end of transmembrane 6 (TM6) of the beta(2) adrenergic receptor (beta(2)AR), adjacent to the G-protein-coupling domain. We demonstrate that this tag reports agonist-induced conformational changes in the receptor, with agonists causing a decline in the fluorescence intensity of fluorescein-beta(2)AR that is proportional to the biological efficacy of the agonist. We also find that agonists alter the interaction between the fluorescein at Cys-265 and fluorescence-quenching reagents localized to different molecular environments of the receptor. These observations are consistent with a rotation and/or tilting of TM6 on agonist activation. Our studies, when compared with studies of activation in rhodopsin, indicate a general mechanism for GPCR activation; however, a notable difference is the relatively slow kinetics of the conformational changes in the beta(2)AR, which may reflect the different energetics of activation by diffusible ligands.
Figures
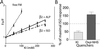
References
-
- Strader C D, Fong T M, Tota M R, Underwood D, Dixon R A. Annu Rev Biochem. 1994;63:101–132. - PubMed
-
- Ji T H, Grossmann M, Ji I. J Biol Chem. 1998;273:17299–17302. - PubMed
-
- Kobilka B. Annu Rev Neurosci. 1992;15:87–114. - PubMed
-
- Gether U. Endocr Rev. 2000;21:90–113. , in press. - PubMed
-
- Palczewski K, Kumasaka T, Hori T, Behnke C A, Motoshima H, Fox B A, Le Trong I, Teller D C, Okada T, Stenkamp R E, et al. Science. 2000;289:739–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

